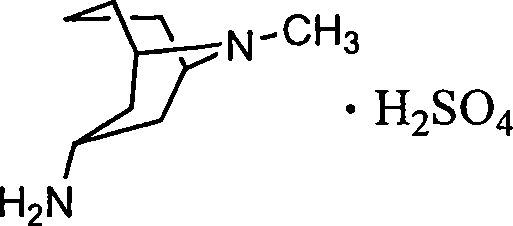
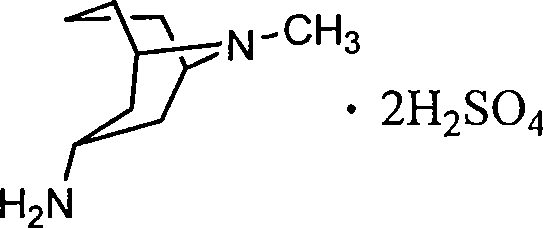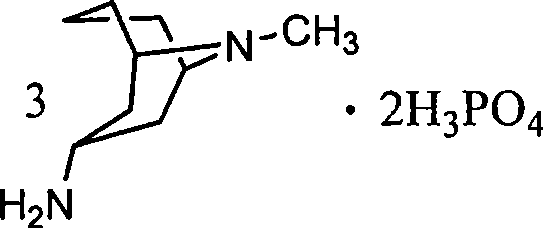3 alpha-high tropine aliphatic amine salt, crystal formation and preparation method
A technology of homotropane dihydrochloride and homotropane, which is applied in the field of six salts of 3α-homotropane and its crystal forms, and can solve the problem of 3α-homotropane that has not been seen. Amine Sulfate and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 10g of 3α-homotropaneamine and 30ml of methanol into a 250ml flask, stir, add 6ml of 12N hydrochloric acid dropwise, cool to room temperature and crystallize. Filtration gave 13.6 g of a white powdery solid product. Yield: 92.2%. In X-ray powder characteristic diffraction spectrum, the diffraction peaks 2θ are 9.90°, 11.648°, 14.643°, 16.118°, 17.748°, 19.568°, 19.956°, 21.765°, 24.950°, 29.407°, 29.789°, 31.198°, 32.345° °, 35.567°, the characteristic peaks in the infrared spectrum appear at 3294, 2868, 2089, 1655, 1613, 1519, 1408, 1322, 1214, 1154, 1038, 963, 693, 589, 503cm -1 place.
Embodiment 2
[0033] Add 15g of 3α-homotropane amine and 80ml of ethanol into a 500ml flask, stir, add 10ml of 12N hydrochloric acid dropwise, cool to room temperature and crystallize. After filtration, 20.2 g of a white powdery solid product was obtained, yield: 91.4%. In X-ray powder characteristic diffraction spectrum, the diffraction peaks 2θ are 9.90°, 11.648°, 14.643°, 16.118°, 17.748°, 19.568°, 19.956°, 21.765°, 24.950°, 29.407°, 29.789°, 31.198°, 32.345° °, 35.567°, the characteristic peaks in the infrared spectrum appear at 3294, 2868, 2089, 1655, 1613, 1519, 1408, 1322, 1214, 1154, 1038, 963, 693, 589, 503cm -1place.
[0034] (this method is the preferred version of the present invention)
Embodiment 3
[0036] Add 15g of 3α-homotropane amine and 100ml of ethanol into a 500ml flask, stir, add 9.5g of sulfuric acid dropwise, cool to room temperature and crystallize. After filtration, 20.3 g of a light red powdery solid product was obtained, yield: 82.8%. In X-ray powder characteristic diffraction spectrum, the diffraction peaks 2θ are 13.394°, 15.144°, 16.487°, 16.964°, 17.691°, 18.109°, 18.616°, 19.553°, 20.002°, 20.719°, 21.115°, 21.654°, 23.596 °, 24.320°, 24.832°, 25.399°, 25.882°, 28.008°, 30.079°, 30.560°, 31.106°, 33.380°, 33.864°, 35.511°, 36.771°, the characteristic peaks in the infrared spectrum appear at 3435, 2930, 1632 , 1535, 1508, 1484, 1456, 1370, 1121, 1035, 967, 619cm -1 place.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com