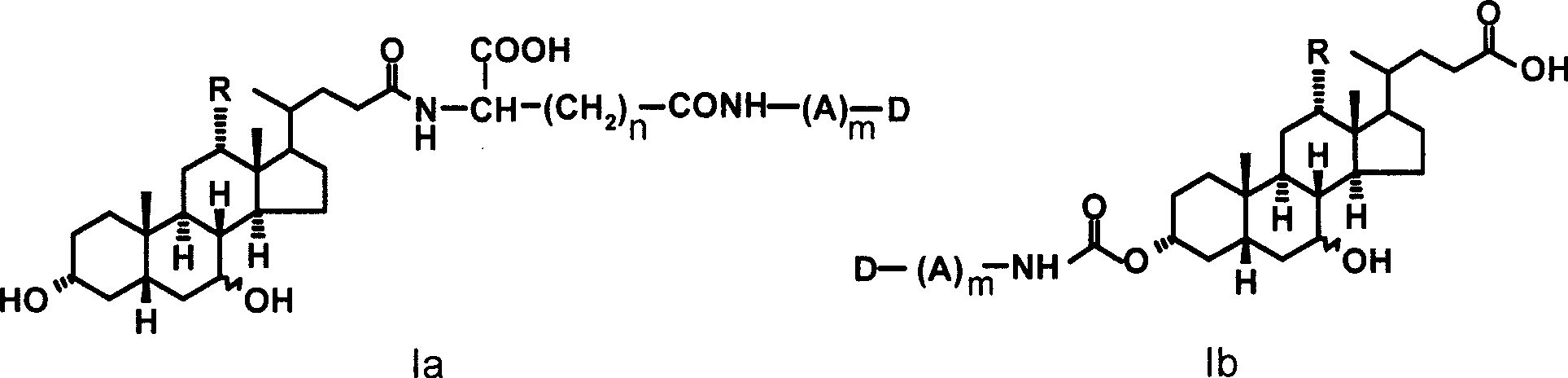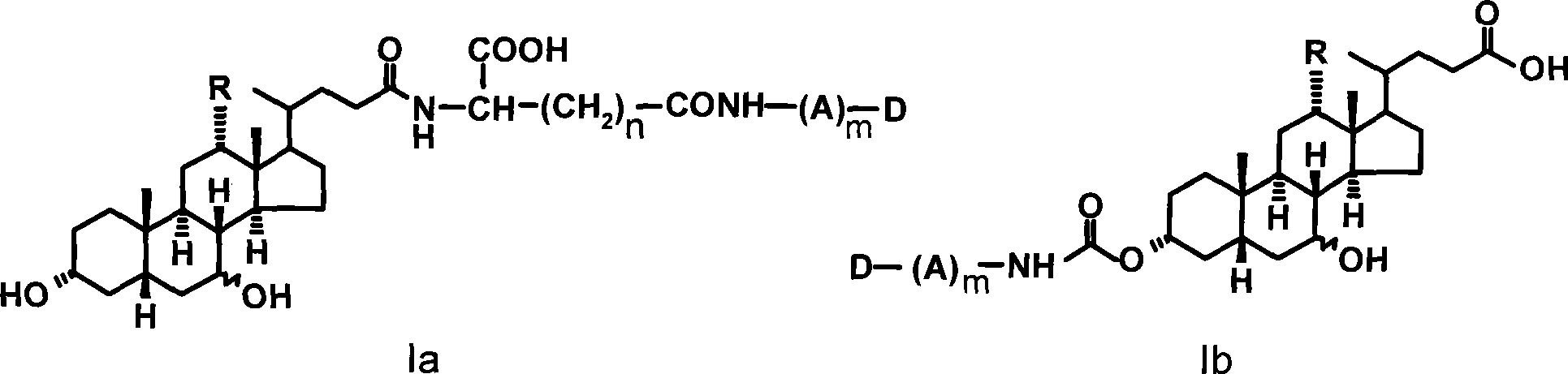Colchicine derivative-bile acid coupling compounds and medical use thereof
A technology of colchicine and derivatives, which is applied in the field of preparation of drugs for the treatment of liver cirrhosis and liver fibrosis, and can solve the problems of small drug loading and complex chemical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of N-cholylglutamic acid-trimethylcolchicinic acid conjugate (Ia-1)
[0029] 1.1 Synthesis of N-choylglutamic acid-α-methyl ester
[0030] 16.1 g (0.1 mol) of glutamic acid-α-methyl ester was dissolved in 160 ml of 1N sodium hydroxide solution, and the mixture was cooled to 0°C. Take 36.7 grams (90 mmol) of cholic acid and dissolve it in 110 milliliters of tetrahydrofuran, and cool it to -15°C in an ice salt bath; add 5.20 milliliters of N-methylmorpholine and 6.02 milliliters of isobutyl chloroformate in turn, React in the bath for 8-10 minutes. The glutamic acid-α-methyl ester solution was added to the reaction solution, stirred under ice bath for 1.5 hours, removed from the ice bath, and stirred at room temperature for 2 hours. After the reaction, the pH was acidified to 3 with 5% citric acid, extracted three times with ethyl acetate, the ethyl acetate layers were combined, washed with 5% citric acid and saturated brine, and dried over anhydro...
Embodiment 2
[0035] Example 2 Preparation of N-ursodeoxycholoylglutamic acid-trimethylcolchicinic acid conjugate (Ia-2)
[0036] With ursodeoxycholic acid instead of cholic acid, refer to the method of Example 1 to obtain compound Ia-2, yield: 72%, TLC: chloroform: methanol = 20: 1, R f = 0.5. MS (m / e): 847.0 (M+1); elemental analysis (C 48 h 66N 2 o 11 ): theoretical value C 68.06%, H 7.85%, N3.31%; experimental value C 68.12%, H 7.74%, N3.40%.
Embodiment 3
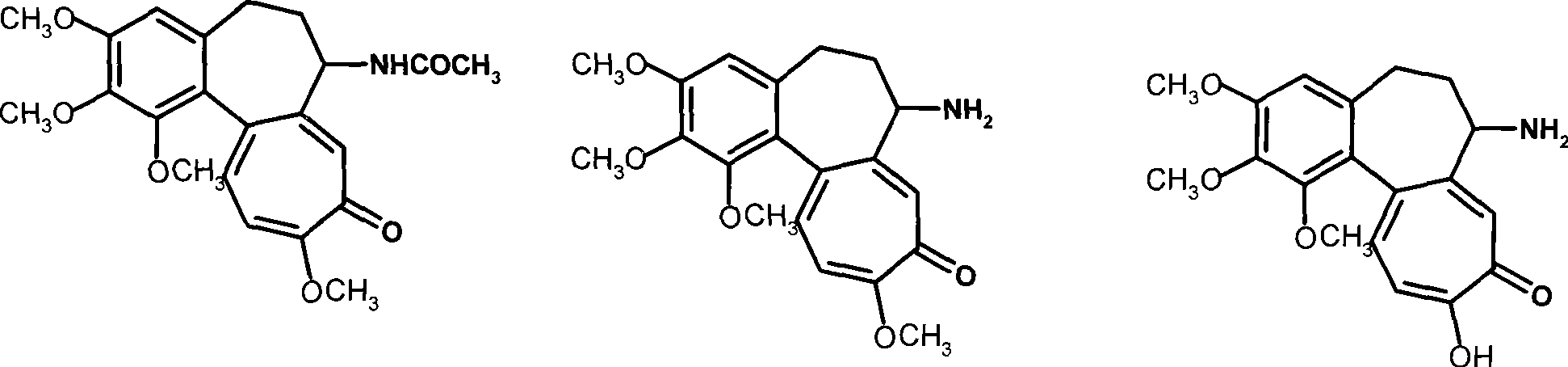
[0037] Example 3 Preparation of N-cholylglutamic acid-deacetylcolchicine conjugate (Ia-3)
[0038] With deacetylcolchicine instead of trimethylcolchic acid, referring to the method of Example 1, compound Ia-3 was obtained, yield: 58%, TLC: chloroform: methanol = 25: 1, R f = 0.3. MS (m / e): 877.3 (M+1); elemental analysis (C 49 h 68 N 2 o 12 ): theoretical value C 67.10%, H 7.81%, N3.19%; experimental value C 67.25%, H 7.70%, N3.24%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com