Inhibitors of protein prenyltransferases
A protein and alkyl technology, applied in the fields of organic chemistry, antitumor drugs, drug combination, etc., can solve the problems of different yields, great differences in reaction conditions, and hindering the adjustment of combinatorial library synthesis methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
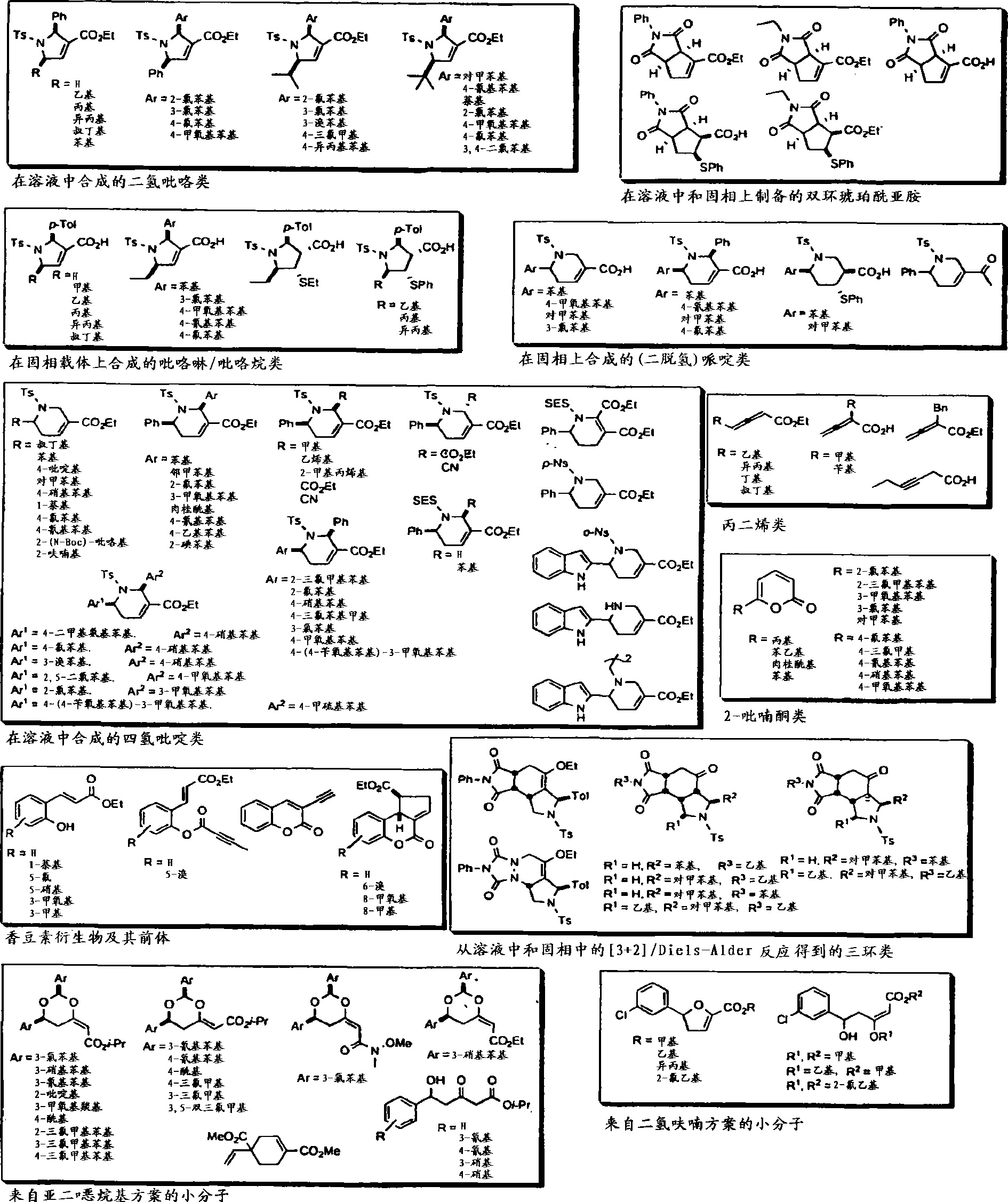
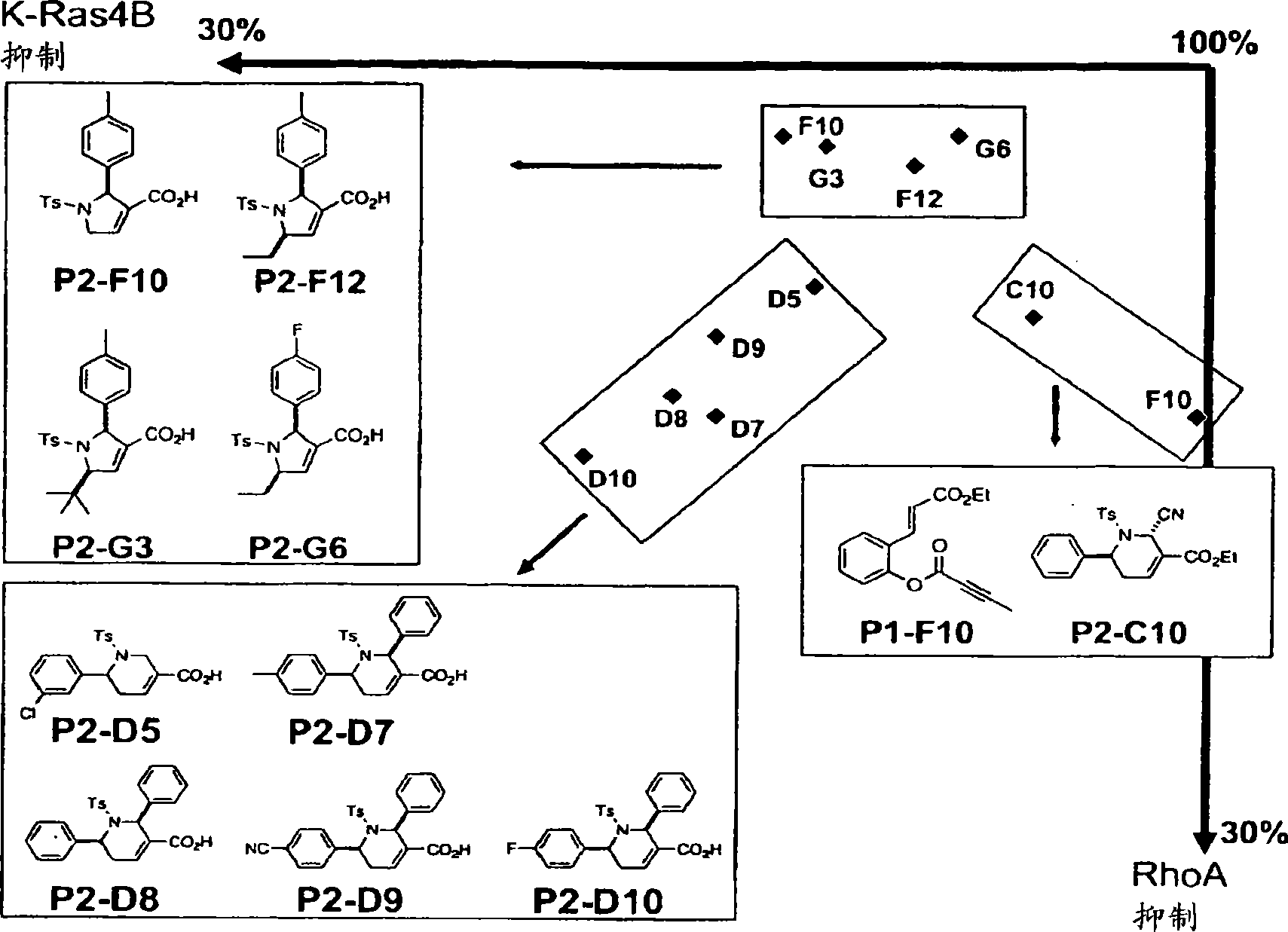
[0246] We report the first example of phosphine catalysis of polymer-bonded allenoate esters and a combinatorial library approach to the development of highly potent inhibitors of the type I protein geranylgeranyltransferase (GGTase-I).
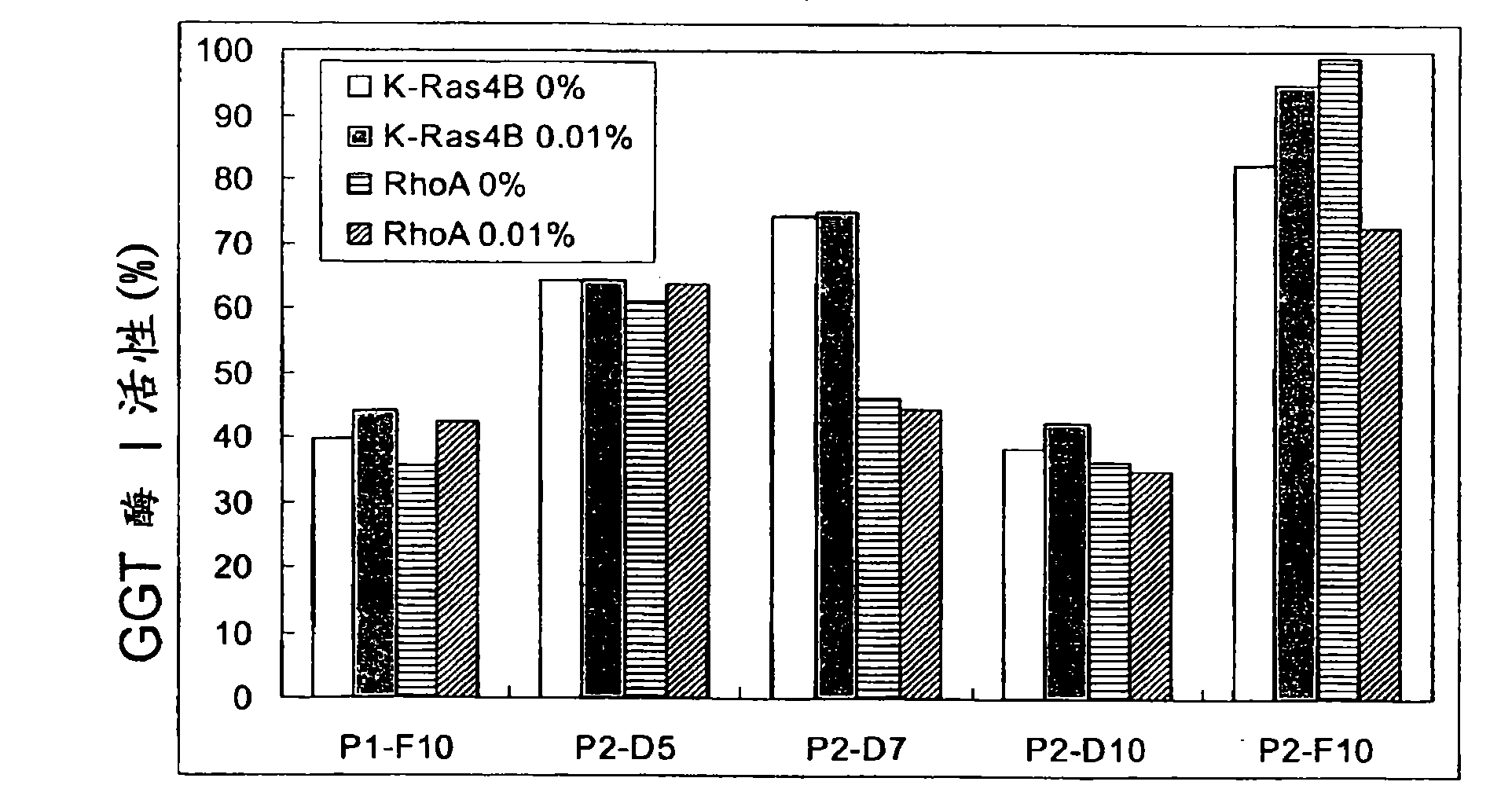
[0247] A panel of 138 heterocycles were screened for their ability to inhibit the activity of human GGTase-I in geranylating K-Ras4B or RhoA geranyl. The purified GGTase-I and its substrate protein K-Ras4B or RhoA, [ 3 H]GGPP, and 138 compounds were incubated together. After 30 minutes, the degree of incorporation of deuterated geranylgeranyl was measured using a scintillation counter.
[0248] A variety of compounds have been identified as GGTIs, including those numbered 1 and 2 below:
[0249]
[0250] The discovery of a promising GGTI lead compound and its modest activity warrants the development of efficient and rapid synthesis and evaluation of similar structures in search of better inhibitors; we conceived a method using SynPhase ...
Embodiment 2
[0271] Such as Figure 20 As shown in A-20C, compounds P3-E5 and P5-H6 specifically inhibit GGTase I. The figure illustrates the effect of P3-E5 and P5-H6 on the enzymatic activity of GGTase-I (A), FTase (B), GGTase-II (C). Different concentrations of the two compounds were added to each enzyme reaction. K-Ras4B, FT enzyme (JENA Bioscience, SanDiego, CA) and [ 3 H] Farnesyl pyrophosphate for FT enzyme (protein farnesyl transferase) assay. Incubate at 37°C for 30 minutes.
[0272] Using RhoA as substrate protein, GGTase-I (JENA Bioscience) and [ 3 H] Geranylgeranyl pyrophosphate for GGTase-I assay. Incubate at 37°C for 30 minutes. Using YPT1, GGTase-II (Calbiochem, San Diego, CA) and REP1 (Calbiochem) as substrate proteins and [ 3 H] Geranylgeranyl pyrophosphate for GGTase-II (or RabGGTase) assay. Incubate at 37°C for 30 minutes. Figure 20 D-20F shows the three-dimensional structures of GGTase I (D), FTase (E) and GGTase II (F), which can be obtained from X-ray diffra...
Embodiment 3
[0274] Such as Figure 21 As shown in A-21D, P3-E5 and P5-H6 compete with substrate proteins but not with GGPP. Figure 21 The panel in shows the double reciprocal plot of the inhibition of GGTase-I by P3-E5 (left) and P5-H6 (right) from the substrate rate curves. The upper graph shows the use of a fixed RhoA protein concentration as well as varying GGPP concentrations. The graph below shows the use of a fixed GGPP concentration as well as varying RhoA protein concentrations. Graphs are plotted using the following symbols: Y-axis: 1 / v, fmol / min. X-axis: 1 / s, mM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com