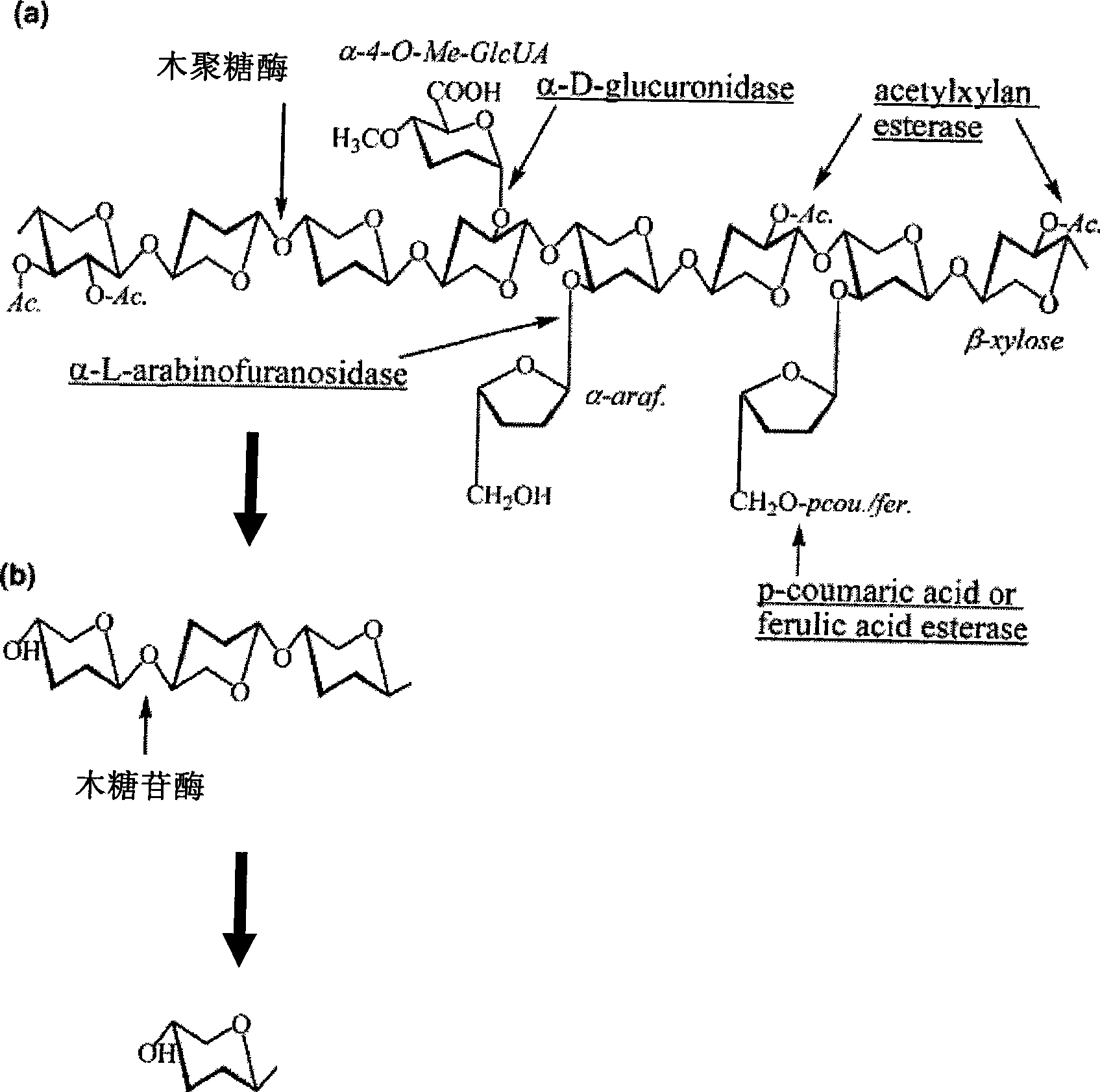
High temperature resistant xylosidase XynB1, gene encoding the enzyme and uses thereof
A technology of xylosidase and encoding gene, applied in the field of xylosidase, can solve problems such as no literature reports, and achieve the effects of good thermal stability and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Extraction of total DNA from Bacillus denitrophilus NG80-2 (CGMCC No.1228)
[0045] The extraction of total DNA from Geobacillus thermodenitrificans NG80-2 (CGMCC No.1228) proved that the gene encoding alcohol dehydrogenase can be isolated from the genome of Geobacillus thermodenitrificans NG80-2. Therefore, in this embodiment, the thermophilic denitrophilic Bacillus NG80-2 obtained from the oil well formation water separation of Guan 69-8 block, Dagang Oilfield, Tianjin, China (it is preserved in the General Microorganism Center of China Microbiological Culture Collection Management Committee) is used. The number is CGMCC No.1228, and the preservation date is October 9, 2004. It has applied for a domestic invention patent and obtained authorization. 3ml of the fresh culture cultured overnight was collected by centrifugation, and the bacteria were suspended in 250μl 50mM Tris buffer (pH8.0), and 10μl 0.4M EDTA (pH8.0) was added, mixed well, incubated at 37℃ for 20min...
Embodiment 2
[0062] Expression, purification and characterization of recombinant xylosidase:
[0063] Insert the above-mentioned recombinant bacteria H1749 monoclonal into 20ml LB medium containing 50μg / ml Kan, culture at 37°C and 180rpm for 12 hours, and then insert the culture into 200ml containing 50μg / ml Kan at 1% (v / v) inoculum. Kan's LB medium was cultivated at 37°C and 220rpm until the OD600 was 0.6, then IPTG was added to a final concentration of 0.1mM, and induced at 37°C and 180rpm for 3 hours. The cells were collected by centrifugation at 5000rpm for 5min, suspended in 50mM Tris-HCl (pH8.0) buffer, disrupted by ultrasonic waves, centrifuged at 14000g for 20min, and the supernatant was the crude extract of alcohol dehydrogenase. This supernatant was purified by chelating agarose gel (Chelating Sepharose) nickel affinity column chromatography, and the enzyme preparation obtained showed a band on SDS-PAGE (see image 3 ). The basic properties of this enzyme system were determined...
Embodiment 3
[0065] 1. measure the specific activity of xylosidase XynB1 of the present invention at different temperatures:
[0066] The xylosidase obtained in the above-mentioned embodiment two is carried out the mensuration of optimal reaction temperature, and specific method is: preparation xylosidase XynB1 reaction system (100 μ l) is: add substrate p-nitrophenol xyloside to final concentration 10mM, A certain concentration of xylosidase XynB1 was added to 100 μl with 50 mM citrate-sodium citrate buffer, pH5.6. Mix well and react in a water bath at 25~100°C for 20 minutes. After the reaction is over, add 400ul of 1M Na 2 CO 3 The reaction was terminated and the light absorbance was measured at 405 nm. Using p-nitrophenol as the standard curve, calculate the amount of enzyme required to generate 1 μmol of p-nitrophenol per minute, which is defined as 1 enzyme activity unit. For measurement results, see Figure 4 . It can be seen from the figure that the optimum reaction temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com