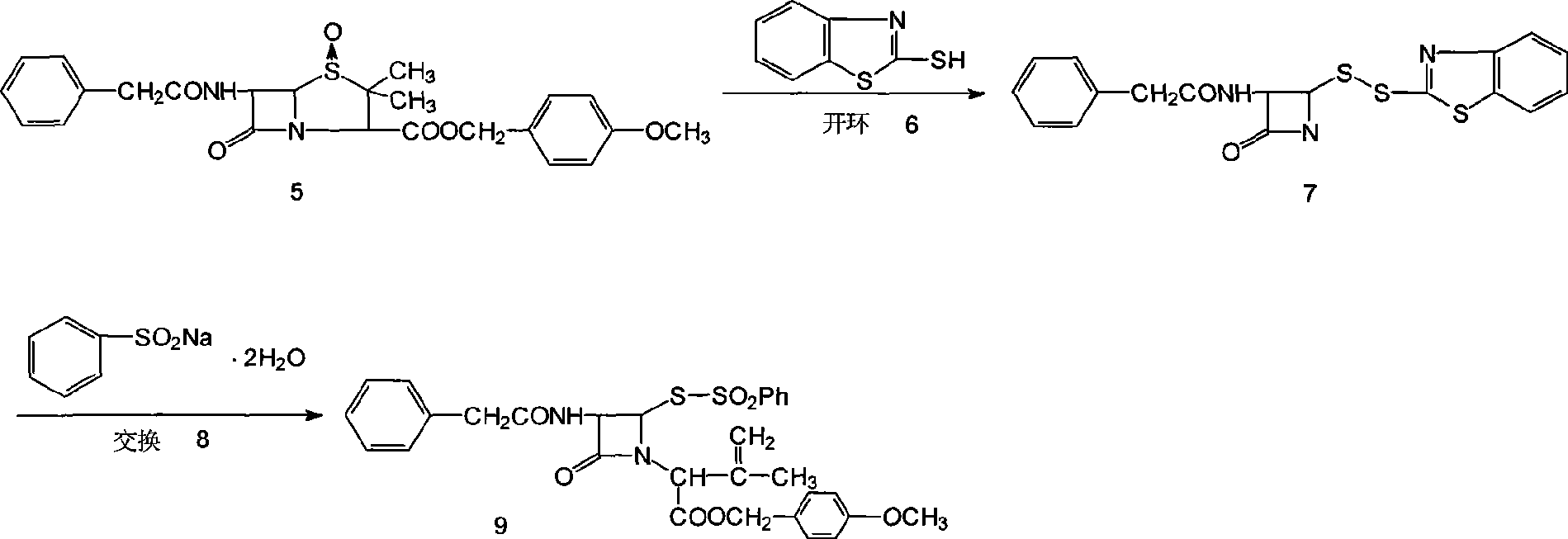
Synthesis method for 7-neophedan-3-chloromethyl cephalosporanic p-methoxy benzyl ester
A technology of chloromethyl cephalosporanic acid and p-methoxybenzyl ester, which is applied in the field of pharmaceutical synthesis, can solve the problems of too many dichlorotrichloro compounds, difficult to realize industrialization, complicated equipment, etc., and achieves simple equipment operation and simplified operation. , the effect of investment reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
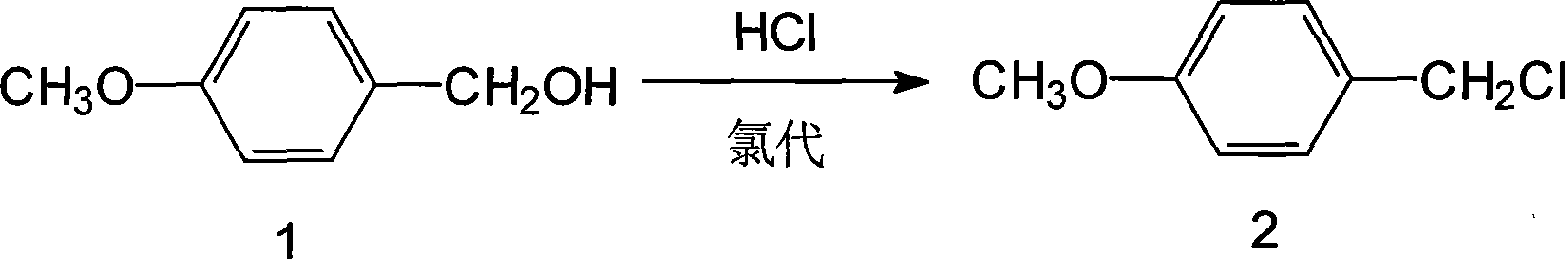
[0073] Example 1 Chloro
[0074] 60g of p-methoxybenzyl alcohol (0.43mmol), add 300ml of toluene, cool to -10°C, add 3g of tetrabutylammonium bromide (0.009mmol), 1g of zinc chloride (0.007mmol), at -10~0°C Next, 150ml of 30% hydrochloric acid (1.444mmol) was added dropwise for about 0.5h, while maintaining the reaction for 1h, the hydrochloric acid layer was removed, and the p-methoxybenzyl chloride solution was set aside.
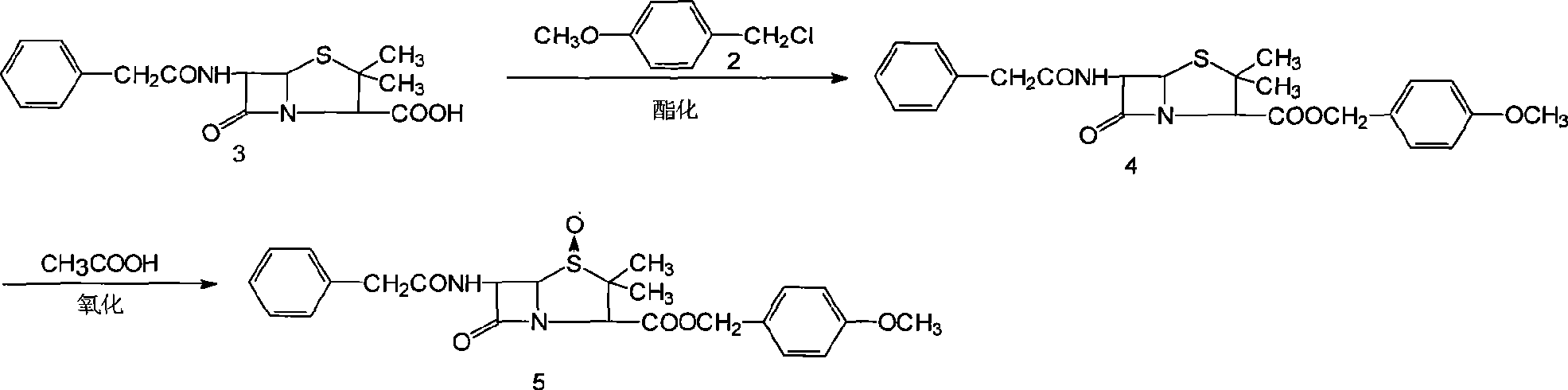
[0075] 1. Esterification
[0076] Add 150g (0.40mmol) of penicillin G potassium, 15g (0.046mmol) of tetrabutylammonium bromide, and 72ml of DMF to the above-mentioned p-methoxybenzyl chloride solution, keep stirring at 30-32°C for 10-12 hours, and add 300ml of water Stand to separate layers, remove the water layer (containing DMF), and leave the penicillin p-methoxybenzyl ester toluene solution for future use.
[0077] 2. Oxidation
[0078] The above toluene solution of penicillin p-methoxybenzyl ester was cooled to -10~-5°C, 150ml (0.43mmol) of 22±2% ...
example 2
[0089] 1. Chlorine
[0090] 60g of p-methoxybenzyl alcohol (0.43mmol), add 300ml of toluene, cool to 0°C, add 3g of tetrabutylammonium bromide (0.009mmol), 1g of zinc chloride (0.007mmol), at 0~10°C, 150ml of 30% hydrochloric acid (1.444mmol) was added dropwise for about 0.5h, while maintaining the reaction for 1h, the hydrochloric acid layer was removed, and the p-methoxybenzyl chloride layer was set aside.
[0091] 2. Esterification
[0092] Add 150g (0.40mmol) of potassium penicillin G (0.40mmol), 15g (0.046mmol) of tetrabutylammonium bromide, and 72ml of DMF to the above-mentioned toluene layer, keep stirring at 25-30°C for 10-12 hours, add 300ml of water and let it stand for stratification. (containing DMF), and the toluene layer of penicillin p-methoxybenzyl ester for later use.
[0093] 3. Oxidation
[0094] The above toluene solution of penicillin p-methoxybenzyl ester was cooled to -20~-10°C, and 150ml (0.43mmol) of 22±2% peracetic acid was added dropwise for about...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com