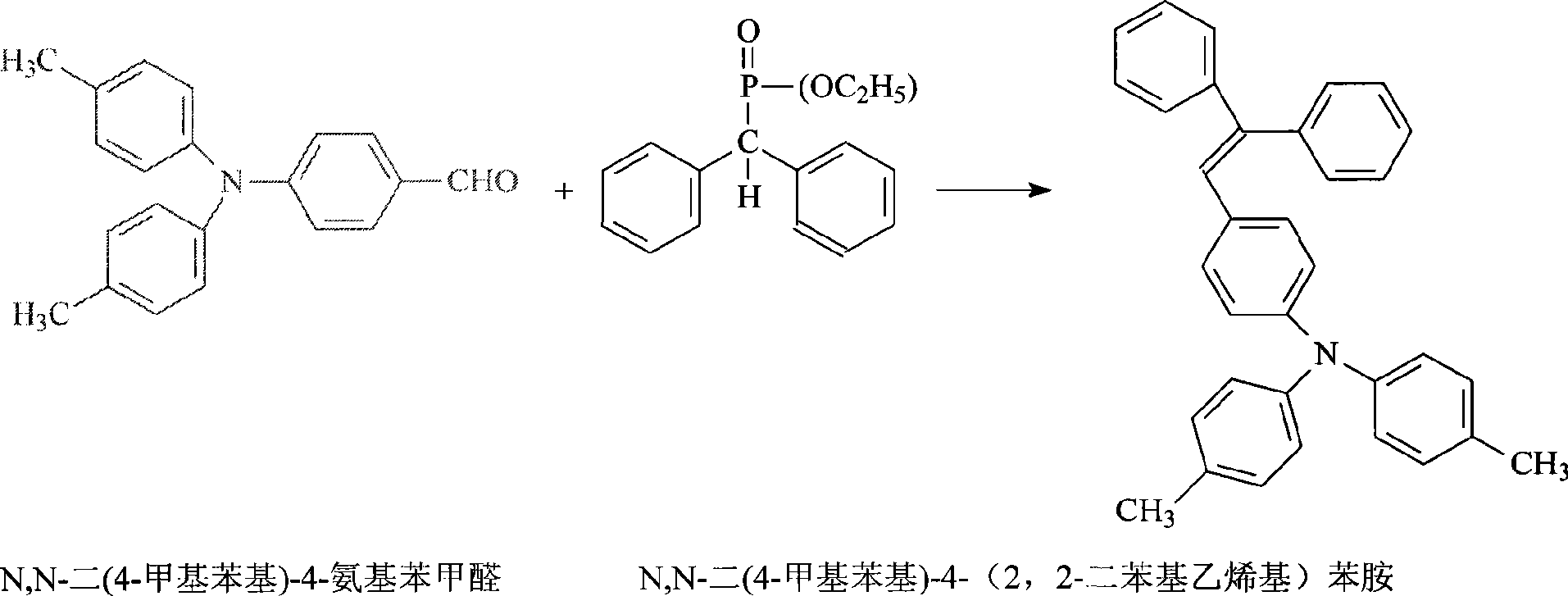
Synthesis of N,N-di(4-methyl phenyl)-4-(2, 2-diphenylethyllene)aniline
A technology of diphenylethylene and methylphenyl, which is applied in the field of N, can solve the problems of being unsuitable for large-scale production, long reaction steps, and low product yield, and achieve products that are easy to purify, have fewer reaction steps, and have high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Synthesis of diethyl diphenylmethylphosphonate:
[0039]Heat hydrobromic acid with a mass percent concentration of 48% to 95°C, add diphenylmethanol, react at 95°C for 2 hours, cool down to 60°C, add toluene, stand still, separate the organic phases, and wash the organic phases. Dry to obtain a toluene solution of diphenylbromomethane; add triethyl acetate to the toluene solution of diphenylbromethane, heat to 156°C for 2 hours, and distill under reduced pressure to obtain crude diethyl diphenylmethylphosphonate , recrystallized with petroleum ether 6 times the weight of the crude product; the molar ratio of hydrobromic acid, diphenylmethanol and triethyl phosphite added was 5.5:1:2. The added sherwood oil weight is 4 times of crude product weight. The high-performance liquid chromatography analysis content was 98.5%, and the yield was 72%.
[0040] 2. Synthesis of N, N-bis(4-methylphenyl)aniline:
[0041] With xylene as solvent, N,N-bis(4-methylphenyl)aniline is ...
Embodiment 2
[0050] 1. Synthesis of diethyl diphenylmethylphosphonate:
[0051] Heat hydrobromic acid with a mass percentage concentration of 48% to 90°C, add diphenylmethanol, react at 90°C for 2 hours, cool down to 40°C, add toluene, stand still, separate layers to take the organic phase, wash the organic phase, and dry , to obtain a toluene solution of diphenylbromomethane; add triethyl phosphite to the toluene solution of diphenylbromethane, heat to 120 ° C for 2 hours, and distill under reduced pressure to obtain diethyl diphenylmethylphosphonate crude product, Recrystallize with petroleum ether 6 times the weight of the crude product; the molar ratio of hydrobromic acid, diphenylmethanol and triethyl phosphite added is 6:1.2:2.5; the yield is 74%.
[0052] 2. Synthesis of N, N-bis(4-methylphenyl)aniline:
[0053] With xylene as solvent, N,N-bis(4-methylphenyl)aniline is produced by reacting aniline and p-iodotoluene under the catalysis of o-phenanthroline, ketone bromide and potassi...
Embodiment 3
[0061] 1. Synthesis of diethyl diphenylmethylphosphonate:
[0062] Heat hydrobromic acid with a mass percentage concentration of 48% to 93°C, add diphenylmethanol, react at 93°C for 1 hour, cool down to 50°C, add toluene, stand still, separate the layers to take the organic phase, wash the organic phase, and dry , to obtain a toluene solution of diphenylbromomethane; add triethyl phosphite to the toluene solution of diphenylbromethane, heat to 140 ° C for 1.5 hours, and distill under reduced pressure to obtain diethyl diphenylmethylphosphonate crude product, Recrystallize with petroleum ether 6 times the weight of the crude product; the molar ratio of hydrobromic acid, diphenylmethanol and triethyl phosphite added is 5.8:1:2; the yield is 73.5%.
[0063] 2. Synthesis of N, N-bis(4-methylphenyl)aniline:
[0064] With xylene as solvent, N,N-bis(4-methylphenyl)aniline is produced by reacting aniline and p-iodotoluene under the catalysis of o-phenanthroline, ketone bromide and po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com