Process for synthesizing liquid crystal compounds containing 1,3-dioxane
A technology for dioxane and liquid crystal compounds, which is applied in the field of compound synthesis, can solve the problems of high operation risk, high price, fire, etc., and achieves the effects of safe operation, easy storage and simple feeding method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
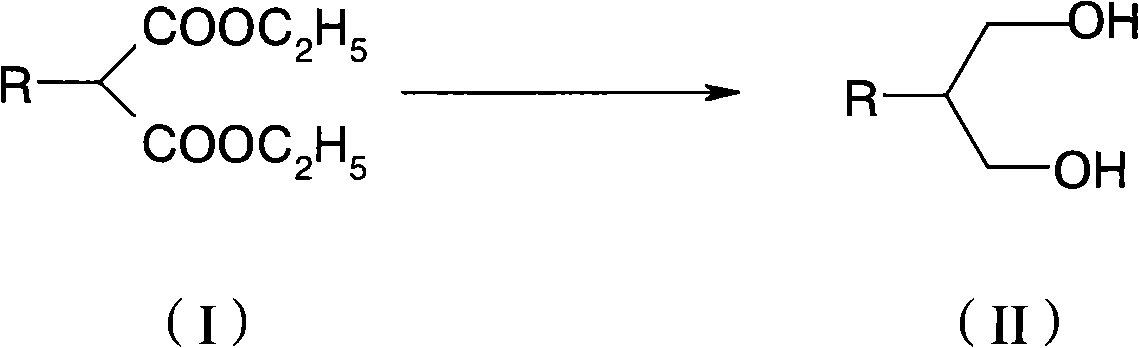
[0037] In this example, the compound of formula (I) is butyl malonate diethyl ester, and butyl propanediol is prepared by hydrogenation reduction reaction to illustrate the hydrogenation reduction reaction of alkyl succinate ethyl ester.
[0038]
[0039] 216g of potassium borohydride, 170g of anhydrous lithium chloride and 1500ml of tetrahydrofuran were mixed, cooled externally with ice water, and a mixed solution of 216g of butyl malonate and 200ml of tetrahydrofuran was added dropwise. After complete addition, the reaction mixture was heated to reflux and reacted for 8-20 hours, monitoring the complete reaction of raw materials. Slowly pour 2kg of crushed ice and 1L of deionized water into the reaction solution, then add 500ml of petroleum ether and stir for 10 minutes, separate the organic phase and extract the water phase with petroleum ether twice, combine the organic phases, wash with deionized water until neutral, and dry , and the solvent was removed under reduced ...
Embodiment 2
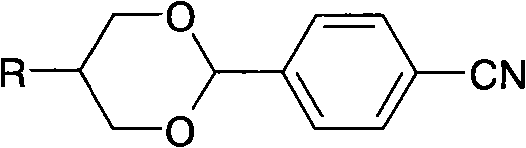
[0046] The obtained alkyl propylene glycol is reacted with the compound of formula (III) to obtain a liquid crystal compound containing 1,3-dioxane.
[0047]
[0048] The 117g butylpropanediol that embodiment 1 obtains, 6g p-toluenesulfonic acid, 115g p-cyanobenzaldehyde and 800ml toluene are mixed. Stir and heat to reflux at 110°C to separate water for 4 hours until no water drops are formed. Cool down to 50°C, wash with water until neutral, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and recrystallize twice with 400ml of absolute ethanol to obtain white crystals of 4-(5-butyl-1,3-dioxane Alk-2-)benzonitrile (1).
[0049] Yield: 162 g (75% of theory), GC: 99.60%, melting point: 44.4°C, clearing point: 34.8°C.
[0050] According to the above-mentioned similar method, the following monomeric liquid crystals can be obtained:
[0051] 4-(5-Ethyl-1,3-dioxane-2-)benzonitrile;
[0052] 4-(5-Propyl-1,3-dioxane-2-)benzonitrile;
[0053] 4-(5-p...
Embodiment 3
[0072] This example prepares butyl propanediol by hydrogenation reduction of the compound of formula (b), in order to illustrate the hydrogenation reduction reaction of ethyl cyclohexyl succinate.
[0073]
[0074] 216g of potassium borohydride, 170g of anhydrous lithium chloride and 1500ml of tetrahydrofuran were mixed, cooled externally with ice water, and a mixed solution of 284g of the compound of formula (2) and 200ml of tetrahydrofuran was added dropwise. After complete addition, the reaction mixture was heated to reflux and reacted for 8-20 hours, monitoring the complete reaction of raw materials. Slowly pour 2kg of crushed ice and 1L of deionized water into the reaction solution, then add 500ml of petroleum ether and stir for 10 minutes, separate the organic phase and extract the water phase with petroleum ether twice, combine the organic phases, wash with deionized water until neutral, and dry , and the solvent was removed under reduced pressure to obtain light yel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Clear point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com