Nitrogen heterocyclic ring substituted arylpropenone compounds, preparation method and application thereof
A technology of aryl acryl ketone and aryl acryloyl chloride, which is applied in the application field of pest control, can solve the problems of limited sources, limited practical application, and difficult synthesis, and achieves good activity, good chemical killing activity, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
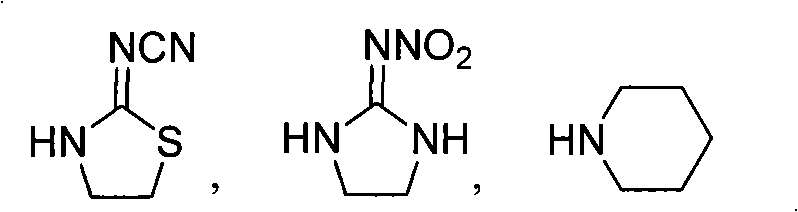
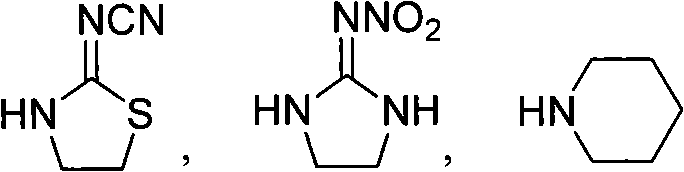
[0030] Example 1. Preparation of (2E)-3-(4-fluorophenyl)-1-(2-cyanoimino-thiazolidin-3-yl)-propenone (A12)
[0031] In a 100 mL three-necked flask, put 1.27 g of 2-cyanoimino-thiazolidine, 1.5 g of piperidine and 30 mL of acetonitrile, stir, add 2.0 g of acetonitrile solution of 4-fluoro-phenylacryloyl chloride, dropwise After completion, the reaction was carried out at 40°C for 5h. The reaction solution was cooled to room temperature, and the solvent was removed under reduced pressure to obtain a red liquid. Then, the product after desolventizing was directly passed through a silica gel column for chromatographic separation, and the eluent was a mixture of ethyl acetate and petroleum ether (V:V=1:10), which was desolvated under reduced pressure to obtain 2.3 g of a white solid. , namely (2E)-3-(4-fluorophenyl)-1-(2-cyanoimino-thiazolidin-3-yl)-propenone (A12), with a yield of 85.0%. That 1 The H NMR spectral data are shown in Table 1.
[0032] Other thiazolidine-containin...
Embodiment 2
[0033] Example 2. Preparation of (2E)-3-(4-fluorophenyl)-1-(2-nitroimino-imidazolidine-1-yl)-propenone (A1)
[0034] Prepared according to the preparation method of Example 1, wherein, 2-nitroiminoimidazolidine is used as the reaction raw material to replace 2-cyanoimino-1,3-thiazolidine, the solvent used is DMF, and pyridine is used to replace piperidine, and the reaction temperature is 80°C, and the reaction time is 8h. The rest of the reaction operation steps are the same as in Example 1.
[0035] Purify the reacted product according to the purification method described in Example 1 to obtain 1.9 g of a white solid product, which is (2E)-3-(4-fluorophenyl)-1-(2-nitroimino-imidazole) Alk-1-yl)-propenone (A1) in 75% weight yield. That 1 See Table 1 for the H NMR spectrum.
[0036] Other substituted aryl acryl ketones containing imidazolidine were prepared in the same way.
Embodiment 3
[0037] Example 3. Preparation of (2E)-3-(4-fluorophenyl)-1-piperidinylpropenone (A23)
[0038] Prepared according to the preparation method of Example 1, wherein, piperidine is used as the reaction raw material to replace 2-cyanoimino-1,3-thiazolidine, the solvent used is toluene, the basic substance used is sodium hydroxide, and the reaction temperature is 100 ° C , the reaction time is 6h.
[0039] Purify the reacted product according to the purification method described in Example 1 to obtain 2.3 g of needle-like solid product, which is (2E)-3-(4-fluorophenyl)-1-piperidinylpropenone (A23) , the yield was 92.0%. That 1 The H NMR spectrum results are shown in Table 1.
[0040] Other piperidine-containing substituted aryl acryl ketones were prepared in the same way.
[0041]The structures, melting points, and hydrogen spectrum data of compounds of formula I are listed in Table-1 below, which were prepared according to various methods similar to those shown in the preceding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com