Process for making monomenthyl esters
A kind of technology of menthyl ester and menthol, applied in the field of monomenthyl ester of dicarboxylic acid, can solve problems such as high toxicity, unrealistic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
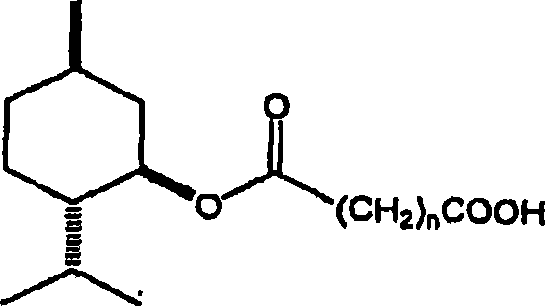
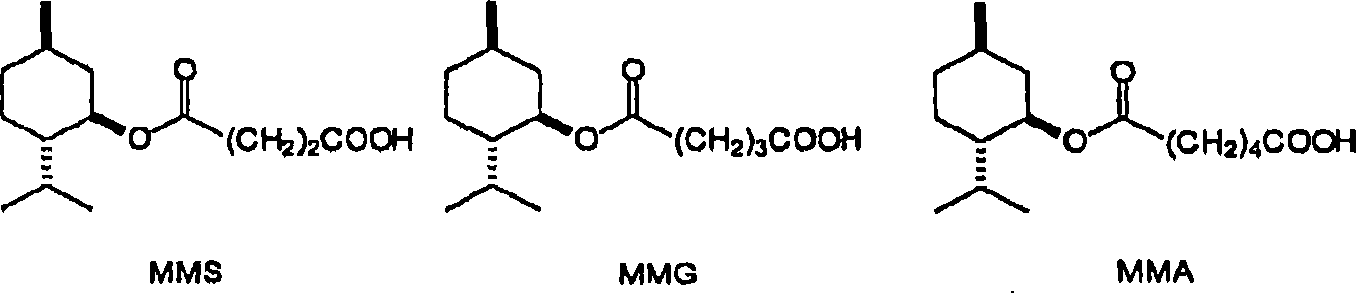
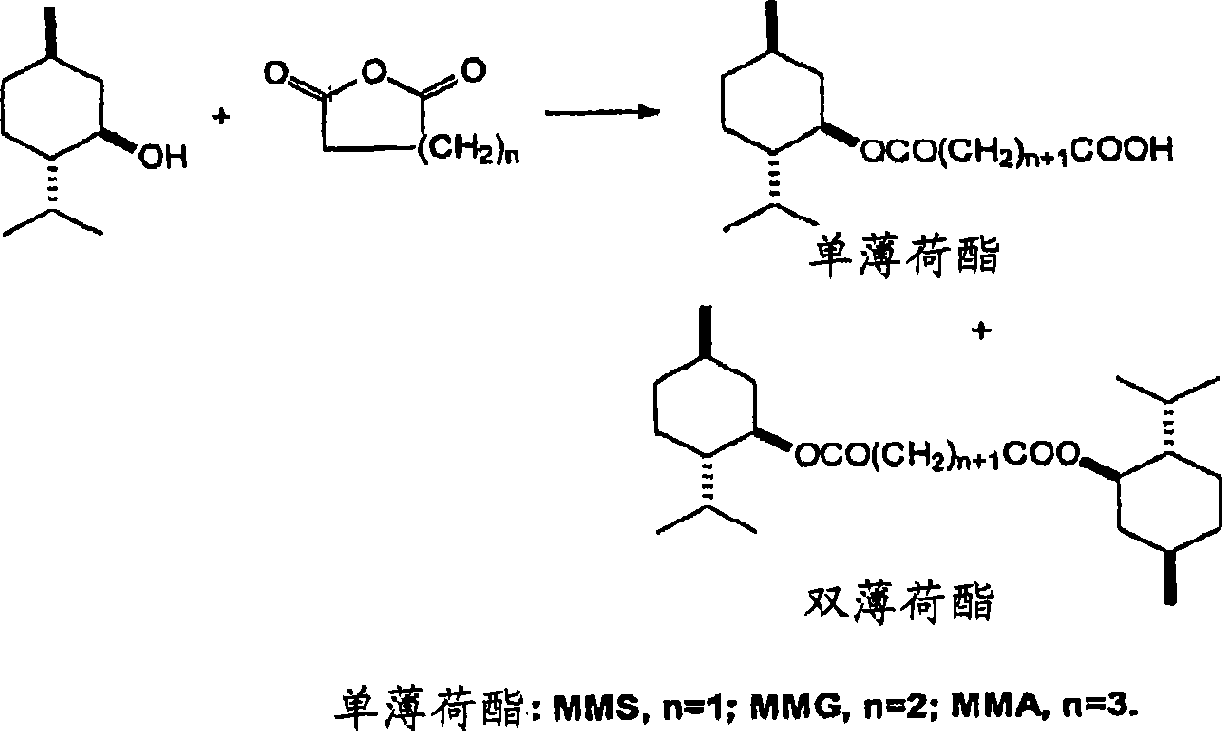
[0013] The method of the present invention reacts menthol with a saturated cyclic anhydride. Menthol suitable for use may have any desired stereochemistry. With three chiral centers, menthol has eight possible stereoisomers. A menthol sample may have several different stereoisomers present. Examples include 1-menthol, d-menthol, d1-menthol (i.e. a racemic mixture of 1-menthol and d-menthol), neomenthol, isomenthol, and isomers of neoisomenthol, and their mixture. 1-menthol, d-menthol, dl-menthol and other isomers are commercially available. 1-Menthol (1) is particularly preferred since it provides a monomenthyl ester with excellent physiological cooling properties.
[0014]
[0015] Suitable anhydrides are saturated cyclic anhydrides. Preferably, the anhydride ring contains 2-4 methylene groups or substituted methylene groups. Examples include succinic anhydride, glutaric anhydride, adipic anhydride, methylsuccinic anhydride, 2-phenylglutaric anhydride, 3-methylglutar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com