Pharmaceutical composition for easing pruritus
A composition and drug technology, applied in the field of pharmaceutical compositions for inhibiting and/or alleviating pruritus, can solve problems such as side effects and unsuitable patients with chronic pruritus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
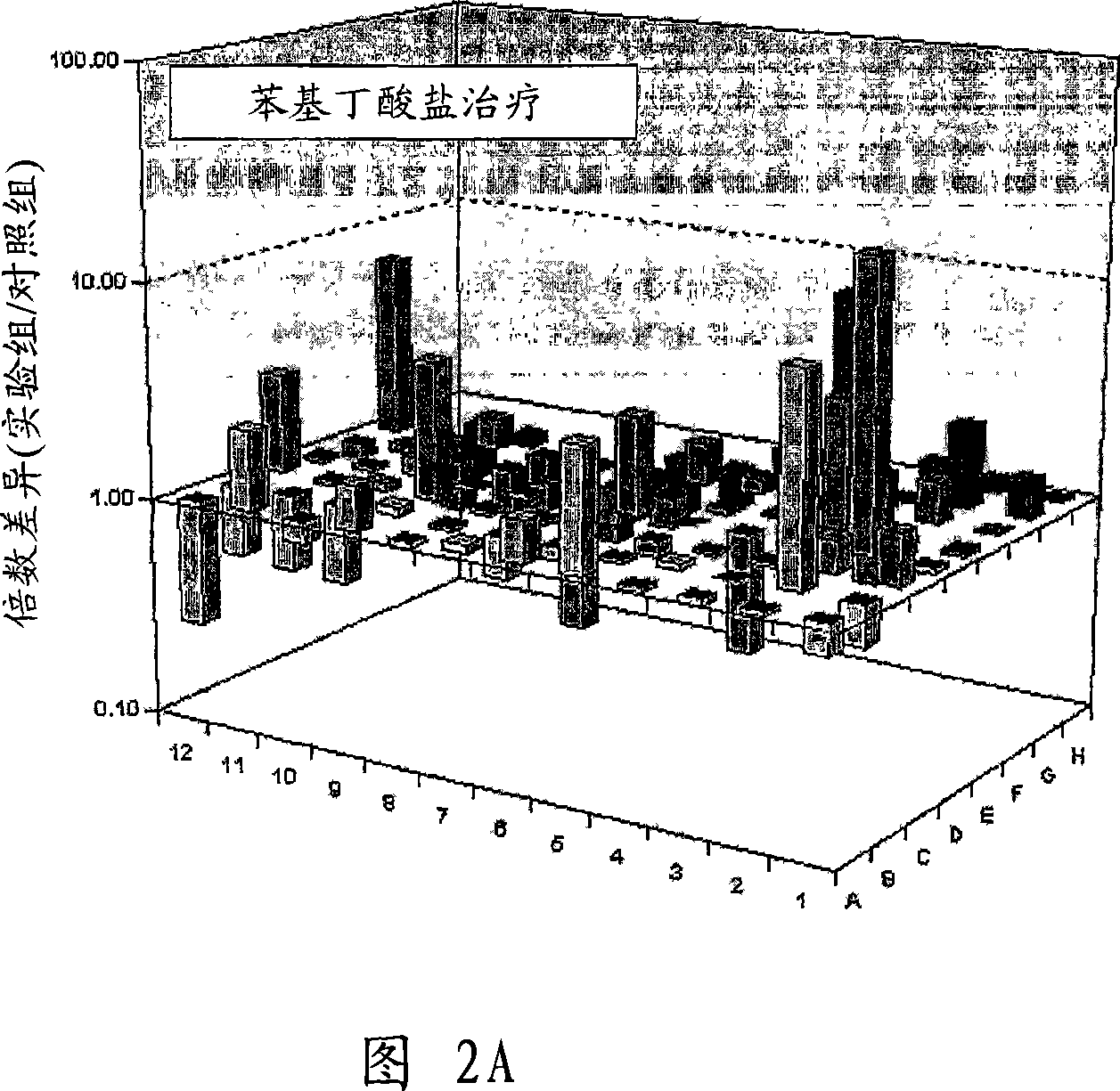
Image
Examples
Embodiment
[0024] 1. Preparation of various types of oily ointments, creams and gels
[0025] A. Preparation of phenylbutyrate oily ointment
[0026] Mix 65 grams of white petrolatum (Riedel-de Haen), 15 grams of cetyl alcohol (Riedel-de Haen), 260 grams of soft paraffin (Merck), 155 grams of liquid paraffin (Merck), and 5 grams of 4-phenylbutyrate (Merck) were mixed in a beaker and heated to 70°C to form a paste. The paste was stirred at 400 rpm for 1 hour and cooled to room temperature.
[0027] B. Preparation of Phenylbutyrate Emulsion
[0028] First ingredient: 70 grams of Tefose 63 , 20 grams of Superpolystate , 10 grams of Coster 5000 , 15 grams of Myriyol 318 , 15 grams of Coster 5088 , and 15 grams of GMS SE (both available from local suppliers) were mixed in a beaker and heated to 70°C.
[0029] Second component: 5.739 grams of sodium 4-phenylbutyrate (Triple Crown America, Inc.), 0.125 grams of methylparaben (Merck), 0.075 grams of propylparaben (Merck) , and 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com