Method for measuring gas hydrate phase balance condition
A gas hydrate and phase equilibrium technology, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems affecting the measurement accuracy, uncertain equilibrium temperature, sensitive hydrate formation, etc., to prevent hydrate blockage, and widely Application background, the effect of high measurement accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Device and method for measuring gas hydrate phase equilibrium conditions
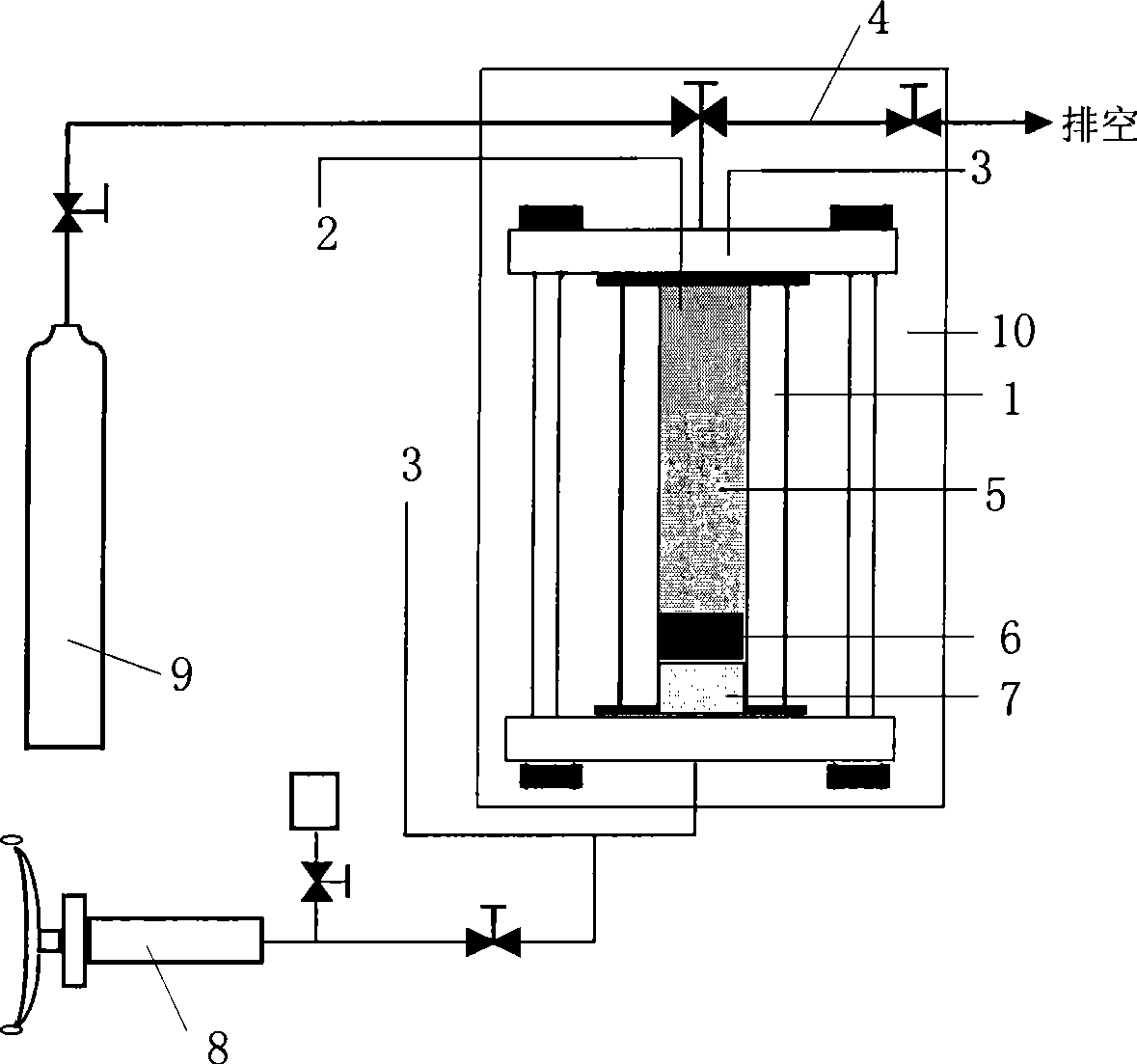
[0036] Please refer to figure 1 As shown, the device includes a reactor 1, the reactor 1 is provided with a temperature measurement system (temperature sensor) 2, a pressure measurement system (pressure sensor) 3, and an exhaust system that can discharge the gas in the reactor 1 4. Wherein, the temperature measuring point of the temperature measuring system 2 is located in the reactor 1, which can directly contact the sample 5 to be tested in the reactor; and the reactor is provided with a piston 6, which connects the pressure transmission medium 7 and the experimental system ( The sample to be tested 5) is separated, and the piston 6 can be pushed by the manual pump 8 through the pressure transmission medium 7 to adjust the system pressure in the reaction kettle. In the device of this embodiment, both the pressure measuring equipment and the reactor are in the constant temperature a...
Embodiment 2
[0039] Embodiment 2: Determination of phase equilibrium conditions of methane+water system hydrate
[0040] In this example, the device in Example 1 is used to measure the phase equilibrium condition of the hydrate in the methane+water system.
[0041] Put pure water in the reaction kettle, maintain the system temperature at 0.5°C, vacuumize, and feed pure methane gas to 9.0MPa, so that a large amount of hydrates will be generated in the reaction kettle. After the hydrate is completely formed, push the piston to compact the hydrate, and then raise the temperature of the system to 4.0°C. Using the existing calculation model, it can be estimated that the equilibrium pressure of the hydrate formation condition of methane in the free water system is 3.80 MPa, the exhaust pressure is reduced to 3.50MPa, the exhaust system is closed, the reactor becomes a closed system, the timing is started, and the pressure change is recorded. After completing the measurement of a set of hydrate t...
Embodiment 3
[0042] Example 3: Determination of hydrate phase equilibrium conditions in methane+SDS aqueous solution (650ppm)+80-100 mesh quartz sand system
[0043] In this example, the device in Example 1 is used to measure the phase equilibrium conditions of hydrates in the methane+SDS aqueous solution (650ppm)+80-100 mesh quartz sand system (porous medium system).
[0044]The reaction kettle is built with sodium dodecylsulfonate (SDS) aqueous solution (SDS concentration 650ppm), and the porous medium sample (80-100 mesh quartz sand) is wrapped with a screen and placed in the reaction kettle to avoid exhaustion. When the medium particles enter the exhaust line. Maintain the system temperature at 0.5°C, vacuumize, and feed pure methane gas to 9.0MPa to form hydrates. Then push the piston to compact the hydrate; raise the temperature of the system to 4.0°C, the equilibrium pressure is estimated to be 3.90MPa at this time, and the exhaust pressure is reduced to 3.50MPa to close the exhaus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com