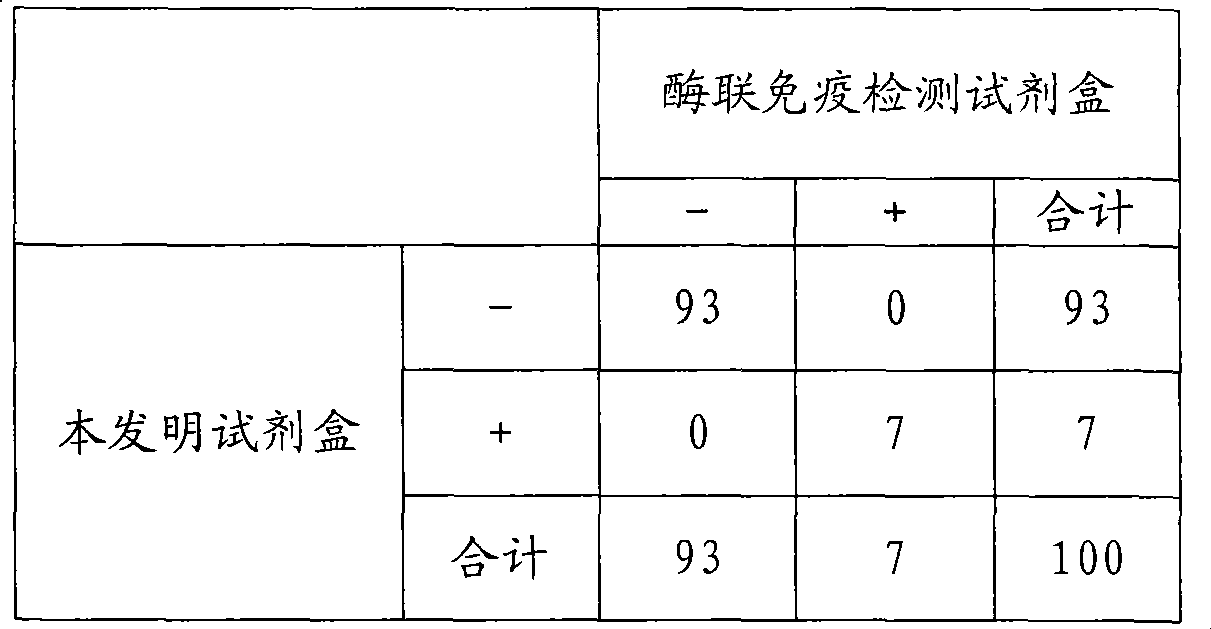
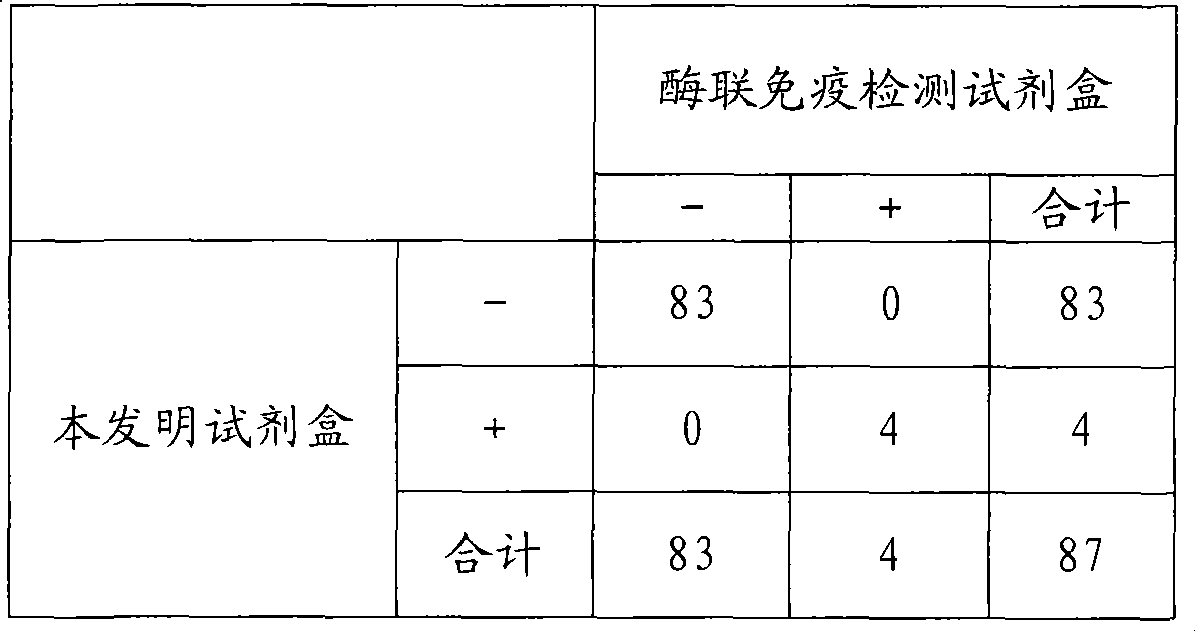
Chemical luminescence immune analysis diagnosis reagent kit detecting Toxoplasma Gondi IgG antibody and preparation method thereof
A chemiluminescence immunoassay, diagnostic kit technology, applied in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, analysis of materials, etc., can solve the problems of toxic substrates, inconvenient operation, low sensitivity, etc. High compliance, easy operation and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Preparation of a chemiluminescent immunoassay diagnostic kit for detecting IgG antibodies to Toxoplasma gondii:
[0069] A, coated plate preparation:
[0070] Coat the microwell plate with 20mmol / L pH7.4 phosphate buffer solution containing 2μg / mL FITC antibody at a volume of 100μL / well. Bovine serum, 5% (weight) sucrose 20mmol / L pH 7.4 phosphate buffer 120μL / well was sealed for 18-20 hours, dehumidified after shaking off the blocking solution, dried at 28°C for 18-20 hours, added a desiccant to seal and Check the sealed aluminum foil bag for air leakage and store at 2-8°C.
[0071] B, preparation of FITC-labeled Toxoplasma gondii antigen:
[0072] a), FITC labeling: dialyze 2 mg of Toxoplasma gondii antigen with 50 mmol / L pH9.6 carbonic acid buffer at 2-8°C, and dissolve FITC in dimethyl sulfoxide (DMSO) to a final concentration of 1 mg / mL , according to the ratio of Toxoplasma gondii antigen:FITC=1mg:150μg, slowly add FITC dimethyl sulfoxide solution into the Toxop...
Embodiment 2
[0096] Preparation of a chemiluminescent immunoassay diagnostic kit for detecting IgG antibodies to Toxoplasma gondii:
[0097] Using magnetic particles as a solid phase carrier, the FITC antibody was coupled to the surface of the magnetic particles by the EDC method, and concentrated with 12% (weight) NaCl, 0.1% (weight) KCl, 400mmol / L pH7.2 Tris-HCl buffer solution Washing solution and the rest were prepared in the same manner as in Example 1 to prepare the Toxoplasma gondii IgG antibody chemiluminescent immunoassay kit.
Embodiment 3
[0099] Preparation of a chemiluminescent immunoassay diagnostic kit for detecting IgG antibodies to Toxoplasma gondii:
[0100] (1), preparation of luminescent substrate solution:
[0101] a), chemiluminescent substrate solution A
[0102] The following formula was used: 1mmol / L luminol, 0.1mmol / L 4-hydroxybiphenyl, 0.01mmol / L 4-iodophenylboronic acid, 50mmol / L pH8.0~10.0 boric acid buffer. Add 0.177g of luminol, 0.017g of 4-hydroxybiphenyl, 0.0025g of 4-iodophenylboronic acid, 2.85g of boric acid, and 1.225g of borax into a clean container and dissolve them in double-distilled water. Adjust the pH value to 8.0-10. Distilled water to dissolve to 1000mL.
[0103] b), chemiluminescent substrate solution B
[0104] The following formula was adopted: 1mmol / L carbamide peroxide, 0.05% (volume) Tween 20, 10mmol / L pH7.0~7.6 phosphate buffer. 0.094g carbamide peroxide, 0.5ml Tween20, 25.79gNa 2 HPO 4 12H 2 O, 4.37g NaH 2 PO 4 2H 2 Add O into a clean container and dissolve wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com