Mango aglycone, preparation purification process and uses thereof
A mangiferin aglycone and purification method technology, which is applied in the fields of organic chemistry, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve the limitation of development and utilization in the field of biomedicine, there is no research report on hypoglycemia, and the distribution of mangiferin aglycone is small. and other problems to achieve the effects of good reproducibility, improved glucose tolerance, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
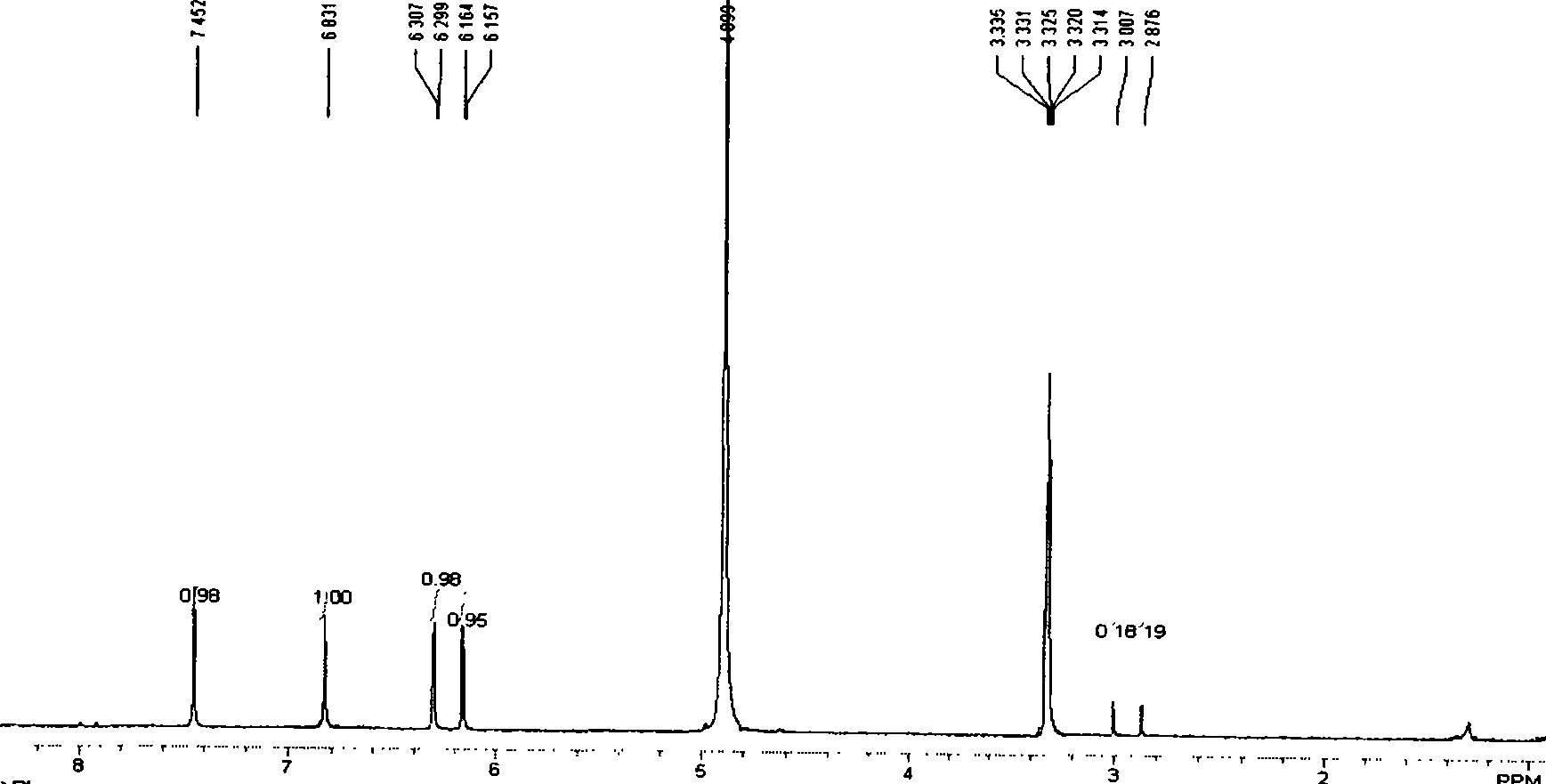
[0024] Example 1 Preparation and purification of mangiferin aglycon
[0025] (1) Heating with a reflux device, first add 12 g of phenol into the three-necked bottle, turn on mechanical stirring, and heat to keep the phenol melted, add 2 g of mangiferin with a grinding funnel, stir until suspended, and cool to room temperature. Measure 22.5ml of HI, and add it dropwise to the three-necked bottle with a constant pressure funnel. Heating with an electric heating pack, stirring mechanically, timing from the beginning of reflux, and reflux for 6 hours.
[0026] (2) Add the solution obtained by reflux for 6h into saturated NaHSO dissolved by ultrasonic 3 , magnetically stirred to fully react. A brown solid was obtained by suction filtration, washed with water several times until the filtrate was colorless. The resulting solid was boiled with 200ml of hot water four times each time, and the filtrate was collected after suction filtration each time. The 800ml filtrates obtained fr...
Embodiment 2
[0031] Example 2 Effect of Mangiferin Aglycone on Blood Glucose in Glucose-Induced Hyperglycemic Mice
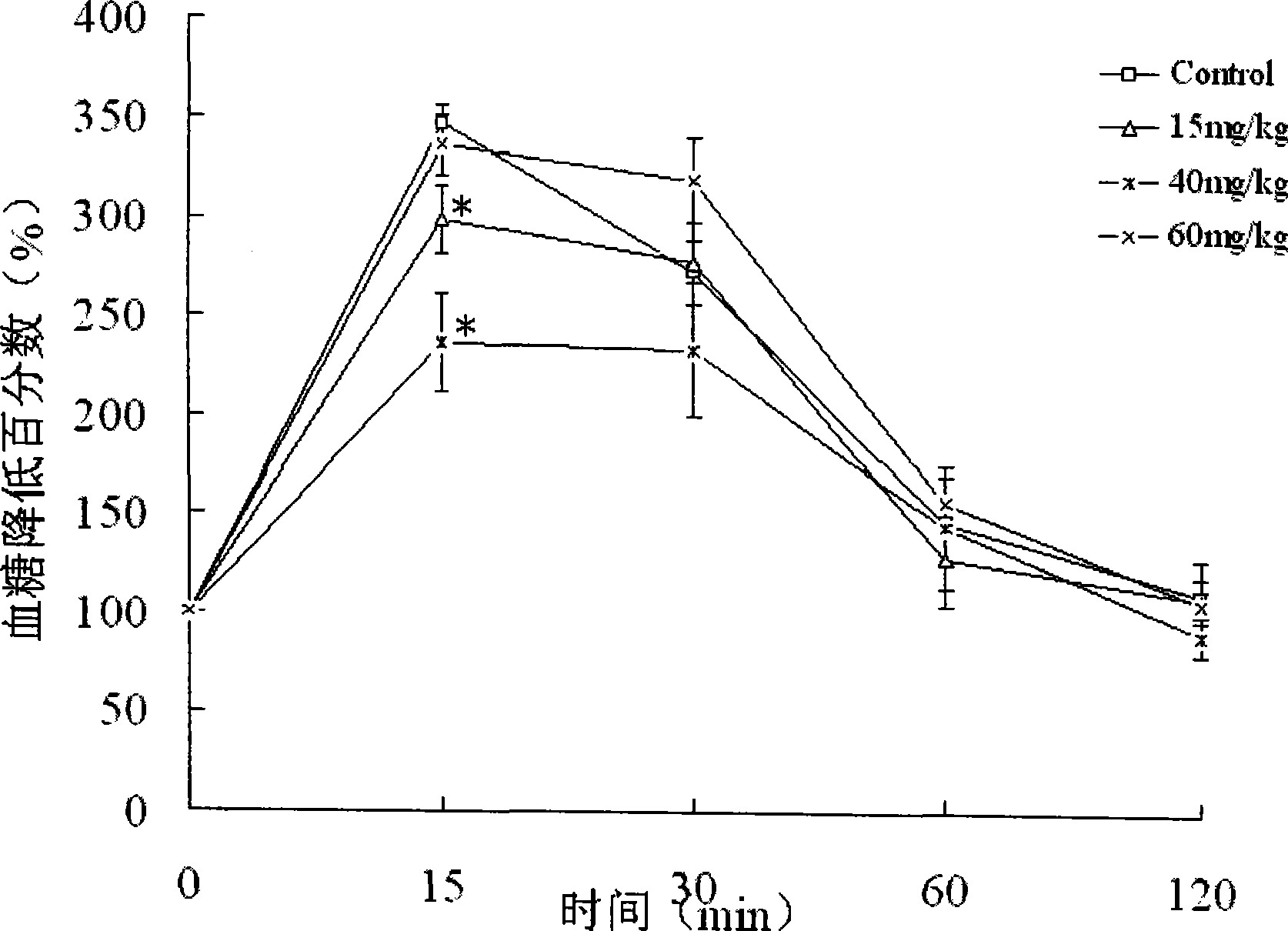
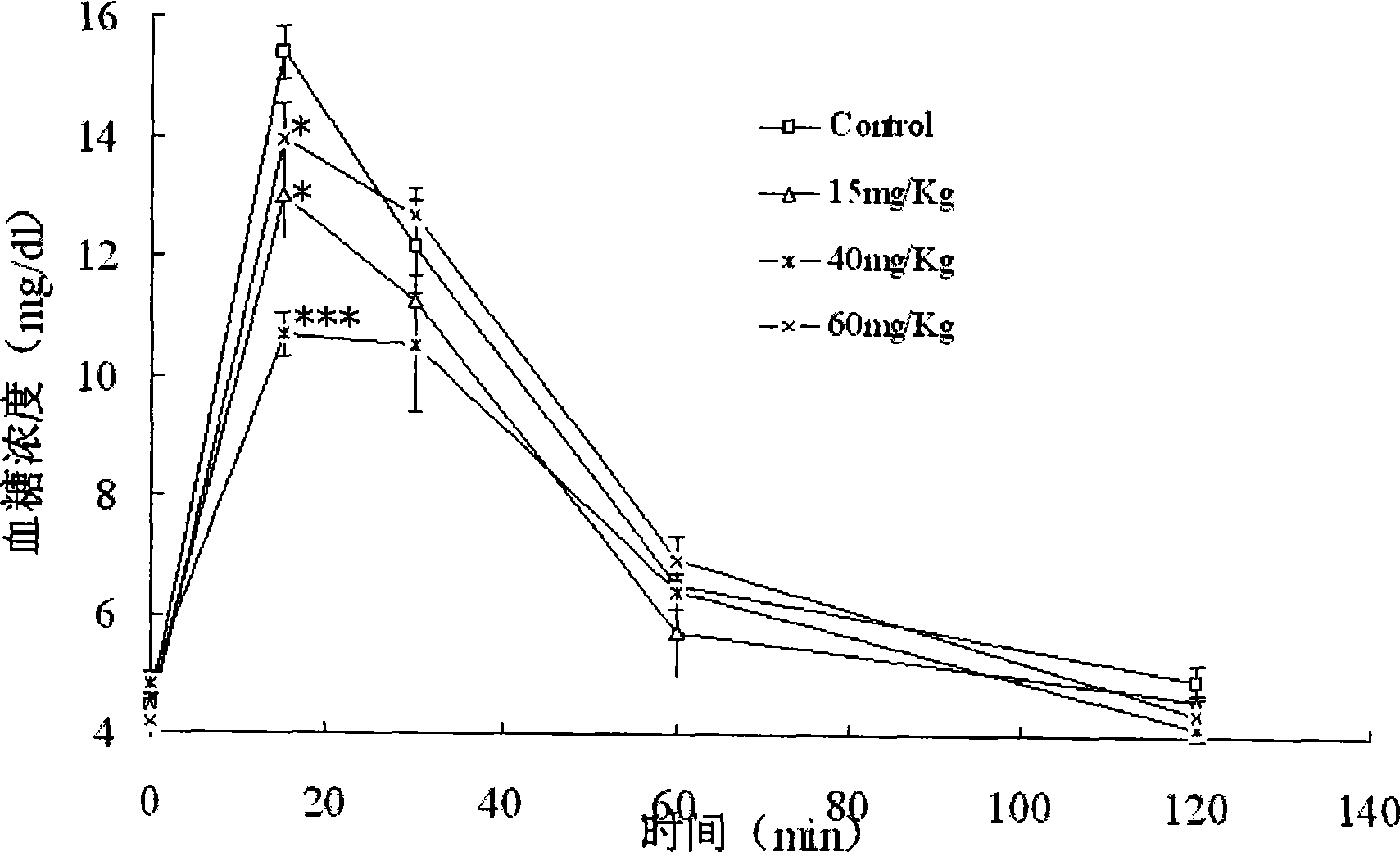
[0032] 48 C57BL / 6 mice were randomly divided into control groups and three doses of administration groups, 12 in every group, and the administration groups were given 15, 40, 60 mg / kg mangiferin aglycone solution (with 0.5% CMC-Na aqueous solution suspension), the control group was given 0.5% CMC-Na aqueous solution. The drug was administered by intragastric administration once a day for 7 days. After fasting for 6 hours after the last administration, 1 g / kg ip.20% glucose solution was administered according to the weight of the mice. Before intraperitoneal injection of glucose (ie basal), 15, 30, 60 and 120 min after ip. glucose, blood was taken from the tail of the mice to measure the blood glucose level. See the experimental results figure 2 , image 3 .
[0033] figure 2 Show that compared with the control group, 15, 40, 60mg / kg mangiferin aglycone has obvious in...
Embodiment 3
[0035] Example 3 Effects of mangiferin aglycone on glucose-induced insulin secretion in normal mice
[0036] 80 C57BL / 6 mice were randomly divided into control groups and three doses of administration groups, 20 in every group, and the administration groups were given 15, 40, 60 mg / kg mangiferin aglycone solution (with 0.5% CMC-Na aqueous solution suspension), the control group was given 0.5% CMC-Na aqueous solution. The administration was administered by intragastric administration once a day for 7 days, and the food was fasted for 6 hours after the last administration. Each dosage group first gets 10 mice to pick eyeball and take blood, as the basal of this group; Every remaining 10 mice, intraperitoneal injection (ip.) gives 20% glucose solution (1g / kg), picks eyeball after 30min Take blood. After standing still, the serum was collected by centrifugation, and the serum insulin content was determined by ELISA method. See the experimental results Figure 4 .
[0037]The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com