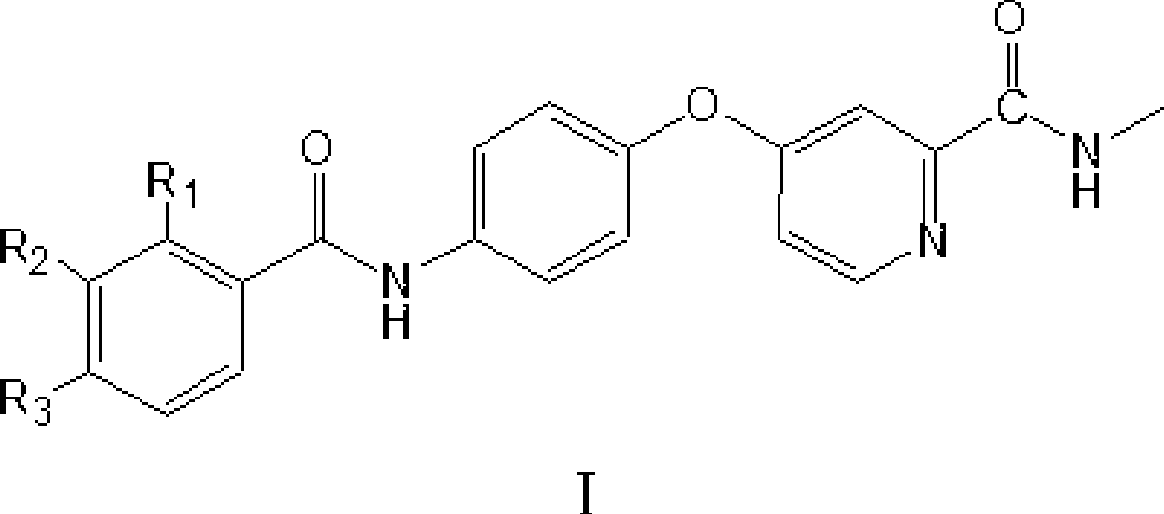
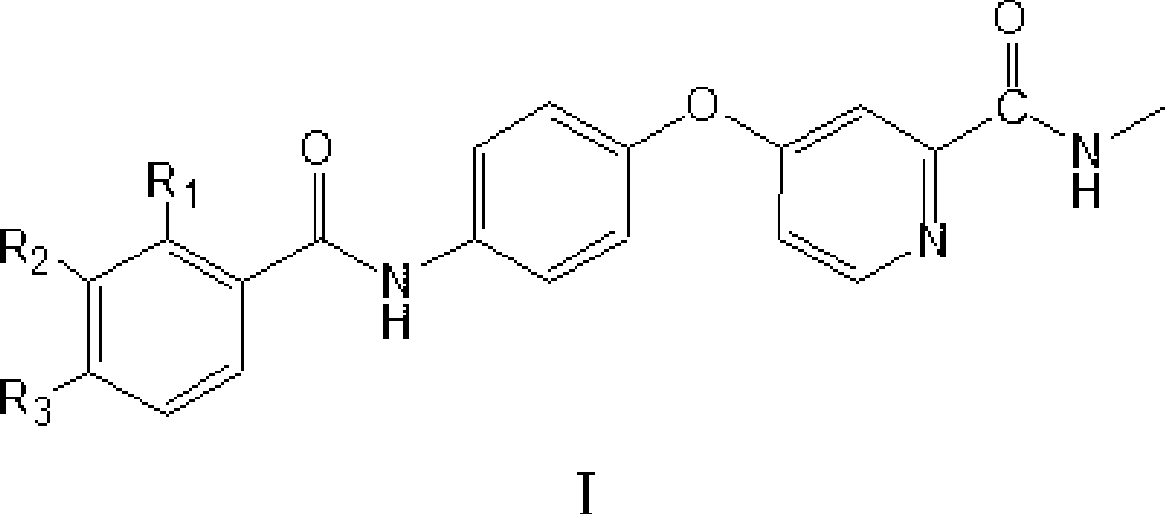
4-(4-benzamido phenoxy)-2-(methylcarbamoyl) pyridine derivatives, preparation method and application thereof
The technology of benzamidophenoxy and benzamidophenoxy is applied in the field of chemical medicine and can solve the problems of killing tumor cells and damaging normal cells, low selectivity, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
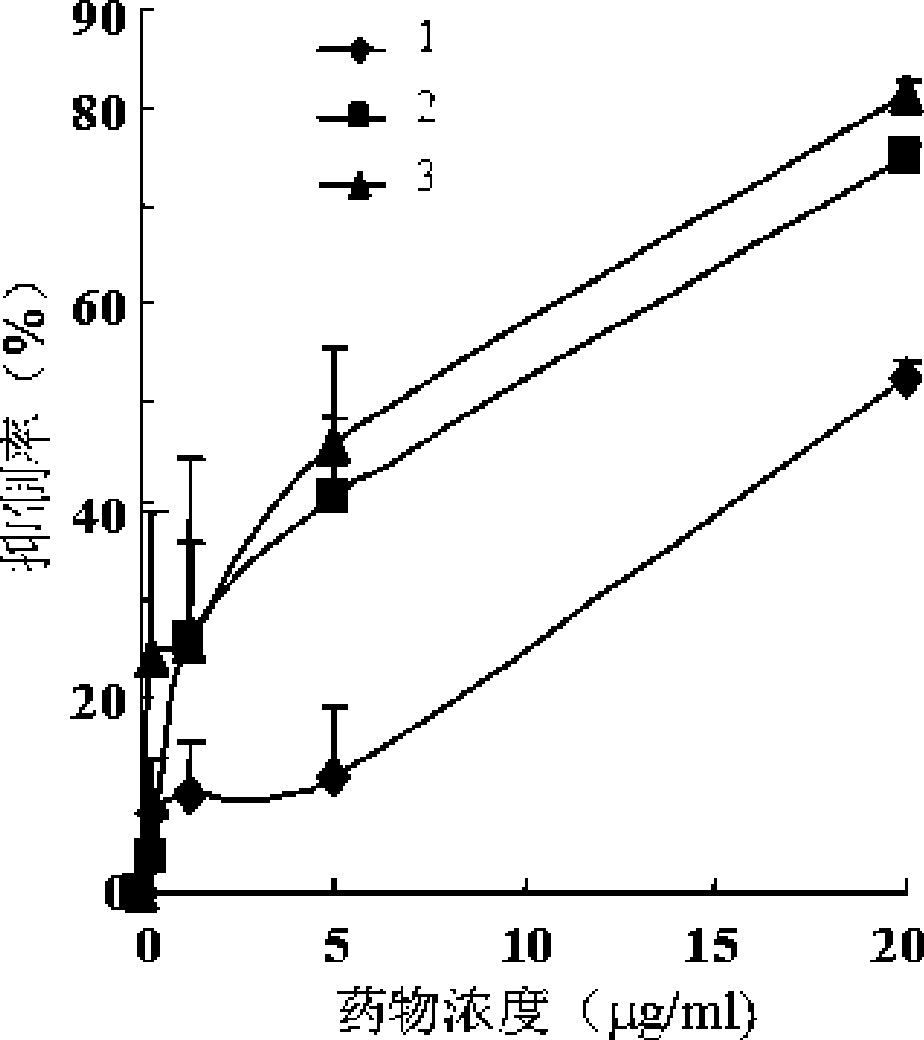
Image
Examples
preparation example Construction
[0034] 4-(4-aminophenoxy)-2-(methylcarbamoyl)pyridine synthetic methods include the following:
[0035] Method 1: Dissolve p-aminophenol in anhydrous DMF at room temperature, then add potassium tert-butoxide, and stir for 2 hours under nitrogen protection. N-methyl-(4-chloro-2-pyridyl)formamide and potassium carbonate were added, and the reaction was heated for 6 hours. Ethyl acetate and water were poured into the reaction solution at room temperature, the organic layer was collected, dried and spin-dried to obtain a crude product, which was recrystallized to obtain brown crystals.
[0036] Method 2: Dissolve N-methyl-(4-chloro-2-pyridyl)formamide in THF, add p-aminophenol, phase transfer catalyst polyethylene glycol 600, sodium hydroxide and 45% sodium hydroxide solution , Reflux for 12h. THF was distilled off, water was added to the residue, filtered, the filter cake was recrystallized with isopropanol, washed with cold isopropanol, and dried under reduced pressure at 40°C...
Embodiment 1
[0038] Example 1 Preparation of 4-(4-aminophenoxy)-2-(methylcarbamoyl)pyridine
[0039] The synthetic route is as follows:
[0040]
[0041] 1, Preparation of 4-chloro-2-pyridinecarbonyl chloride hydrochloride
[0042] Prepared from 2-formic acid pyridine according to the existing published method, for example: in a dry reaction flask, 2-formic acid pyridine (30.00g, 0.244mmol) and NaBr (4.00g, 0.040mmol) were mixed, then added to chlorine In benzene (40ml), when the suspension was heated to 50°C, thionyl chloride (61ml, 0.840mmol) was slowly added to make the released gas (SO 2, HCl) are effectively controlled. The mixture was heated to 85°C and stirred for 19 hours. After the reaction is completed, cool to 20° C., and remove excess thionyl chloride and chlorobenzene mixture by rotary evaporation. Toluene (65.70g, 75.5ml) was added, and the remaining thionyl chloride and a large amount of chlorobenzene were removed by rotary evaporation again. The obtained crude produc...
Embodiment 2
[0049] Example 2 Preparation of 4-(4-aminophenoxy)-2-(methylcarbamoyl)pyridine
[0050] Dissolve p-aminophenol (0.70 g, 6.40 mmol) in 7 ml of anhydrous DMF at room temperature, add potassium tert-butoxide (0.72 g, 6.40 mmol) and stir for 2 hours under nitrogen protection. N-methyl-(4-chloro-2-pyridyl)formamide (0.50g, 2.90mmol) and potassium carbonate (0.20g, 1.40mmol) were added, and the reaction was heated for 6 hours. The reaction solution was poured into the mixed solution (ethyl acetate 200ml+water 20ml) at room temperature, the organic layer was collected, and the solvent was removed to obtain a crude product, which was separated by column chromatography to obtain 0.41 g of a brown-black solid (57.1% yield).
[0051] 1 H NMR (400MHz, CDCl 3 ): 3.0 (d, J = 5.2Hz, 3H, -NHCH 3 ), 3.7 (br, s, 2H, -NH 2 ), 6.7(d, J=8.8Hz, 2H), 6.9(m, 3H), 7.67(d, J=2.4Hz, 1H), 8.0(s, 1H, NH), 8.3(d, J=6Hz, 1H)
[0052] MS-ESI(m / z): 244.4(M+1), 266.4(M+23)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com