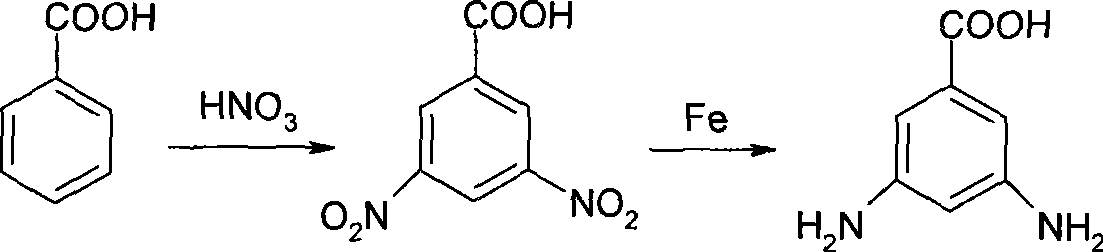
3,5-diaminobenzoic acid preparation method
A technology of m-diaminobenzene and dinitrobenzoic acid, which is applied in the field of preparation of m-diaminobenzoic acid, can solve the problems of waste residue wastewater pollution, low yield, poor product quality, etc., and achieve high yield and short process route , no effect of three wastes pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Put 300 ml of methanol as a solvent, 50 g of raw material 3,5-dinitroformic acid, and 0.25 g of Ni-Al type nickel as a catalyst into a 1-liter stainless steel high-pressure reactor, heat up at 20°C and feed hydrogen, control the hydrogen pressure at 2 MPa, react for 10 hours, discharge After pressing, the material is discharged and filtered, and the filtrate is separated from methanol to obtain the product m-diaminobenzoic acid. The content of chromatographic analysis is 95%, and the yield is 96%. The catalyst was washed with methanol for later use.
Embodiment 2
[0023] Use 300 milliliters of ethanol as solvent, get 50g raw material 3,5-dinitroformic acid, Ni-Al type nickel 3.5g, control hydrogen pressure 3MPa, react at 150 ℃ of temperature for 3 hours, operate with embodiment 1, obtain the product between two Aminobenzoic acid, the content of chromatographic analysis is 95%, and the yield is 97%.
Embodiment 3
[0025] Take 7.1gPaCl 3 , 304 g (NO 3 ) 3 ·6H 2 O was dissolved in 1.2 liters of distilled water and 20 milliliters of concentrated hydrochloric acid, heated to 50°C, and 500 milliliters of 30% sodium hydroxide was added for precipitation reaction. After 4 hours of reaction, solution 1 was obtained; distilled water was added to 90 grams of activated carbon, and the volume ratio was 1 : 2, stirring at 50°C until viscous slurry 2; adding solution 1 into slurry 2, reacting, reducing by passing through hydrogen, washing with methanol and water, and drying in vacuum at 120°C to obtain a catalyst activated carbon carrier.
[0026] Add 0.1 mole m-dinitrobenzoic acid (chemically pure), 100ml methanol (chemically pure) and 0.25 g of catalyst activated carbon carrier into the autoclave, feed hydrogen into the autoclave for 2 times, then pass into 0.3 mole of hydrogen, The reaction was carried out at a pressure of 1MPa, and the temperature was controlled at 80°C. After the system no lo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com