Medicine combination for treating viral hepatitis and quality control method thereof
A technology for viral hepatitis and composition, applied in the field of pharmaceutical composition, preparation method and quality control, can solve the problems of addiction, obvious side effects, drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0037] Experimental Example 1: Pharmacodynamic test of the drug group on liver protection, enzyme reduction, choleretic, jaundice reduction, phagocytosis and clearance
[0038] Drug group I: get the pharmaceutical composition mixture prepared in Example 2 of the present invention
[0039] Pharmaceutical group II: take the pharmaceutical composition mixture prepared in Example 13 of the present invention
[0040] Control group: commercially available Qingre Shugan mixture.
[0041] 1. Hepatoprotective and enzyme-lowering effects
[0042] 1. Protective effect on acute liver injury
[0043] Take 84 Kunming mice, weighing 18-20 g, half male and half male, and randomly divide them into 7 groups. The first group: the normal control group, fed with the same amount of drinking water every day; the second group: the model group, fed with the same amount of drinking water every day; the third group: the drug group I, fed with the drug group I liquid 30ml ( Equivalent to crude drug 9...
experiment example 2
[0068] Experimental Example 2 Excipient screening experiment
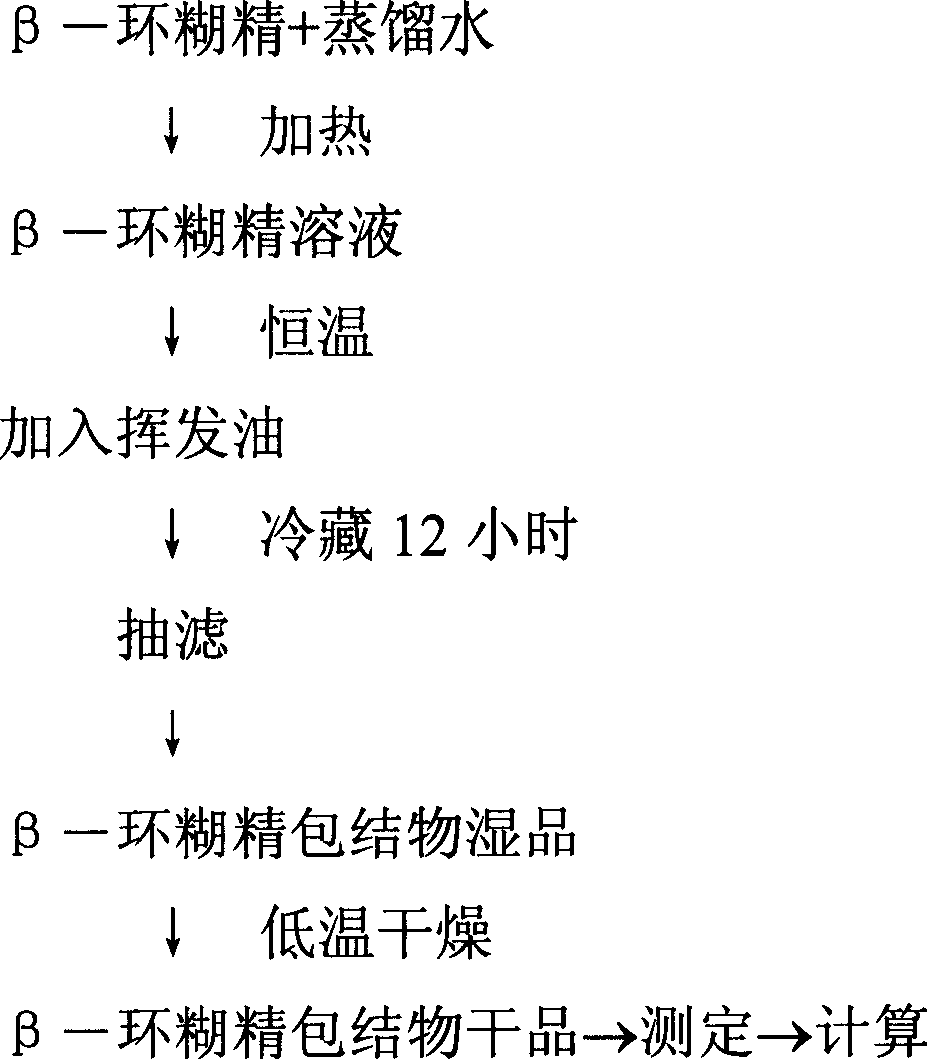
[0069] Optimization of Inclusion Process Conditions of Volatile Oil β-Cyclodextrin
[0070] Because the volatile oil is easy to lose, if the volatile oil is directly added, it will be lost for a long time or slightly heated, thereby reducing the content and affecting the curative effect of the medicine. In order to solve this problem, the method of β-cyclodextrin inclusion was adopted, and the conditions of inclusion were optimized by orthogonal experiment.
[0071] See the accompanying drawings for the experimental procedure.
[0072] Inclusion by saturated aqueous solution method: Weigh a certain amount of β-cyclodextrin, add appropriate amount of distilled water, and heat it in a water bath at 70°C to make a 5% aqueous solution of β-cyclodextrin, at different temperatures (20°C, 40°C, 60°C) stirring at a speed of 2500 rpm, adding a certain amount of volatile oil, stirring fully for a certain period of time, to...
experiment example 3
[0083] Experimental example 3 identification test of capillary
[0084] Preparation of blank samples According to the medicinal taste and dosage ratio of the medicinal group of the present invention, a group of medicines not containing capillary root, white peony root, angelica sinensis, salvia miltiorrhiza, and knotweed was prepared respectively, and blank preparations were made according to the preparation process.
[0085] Preparation of blank solution Take the daily dose of blank sample 14 / 1000 of the pharmaceutical composition preparation capillary, add 20ml of water to dissolve, extract 2 times with ethyl acetate, 30ml for the first time, 20ml for the second time, and combine the ethyl acetate solution , evaporated to dryness in a water bath, and the residue was dissolved by adding 1ml of ethyl acetate as a blank solution.
[0086] Preparation of the sample solution Take the same amount of samples of the drug group and substance, and prepare the sample solution according...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com