Cistanche oligosaccharide, preparation and application thereof
A technology of Cistanche deserticola and total oligosaccharides, which is applied in the preparation of sugar derivatives, chemical instruments and methods, oligosaccharides, etc., can solve the problems of intestinal wall cell function and morphological changes, large intestinal epithelial damage, and complex extraction processes, and achieve enhanced The effect of intestinal peristalsis, promotion of fecal excretion, and elimination of purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
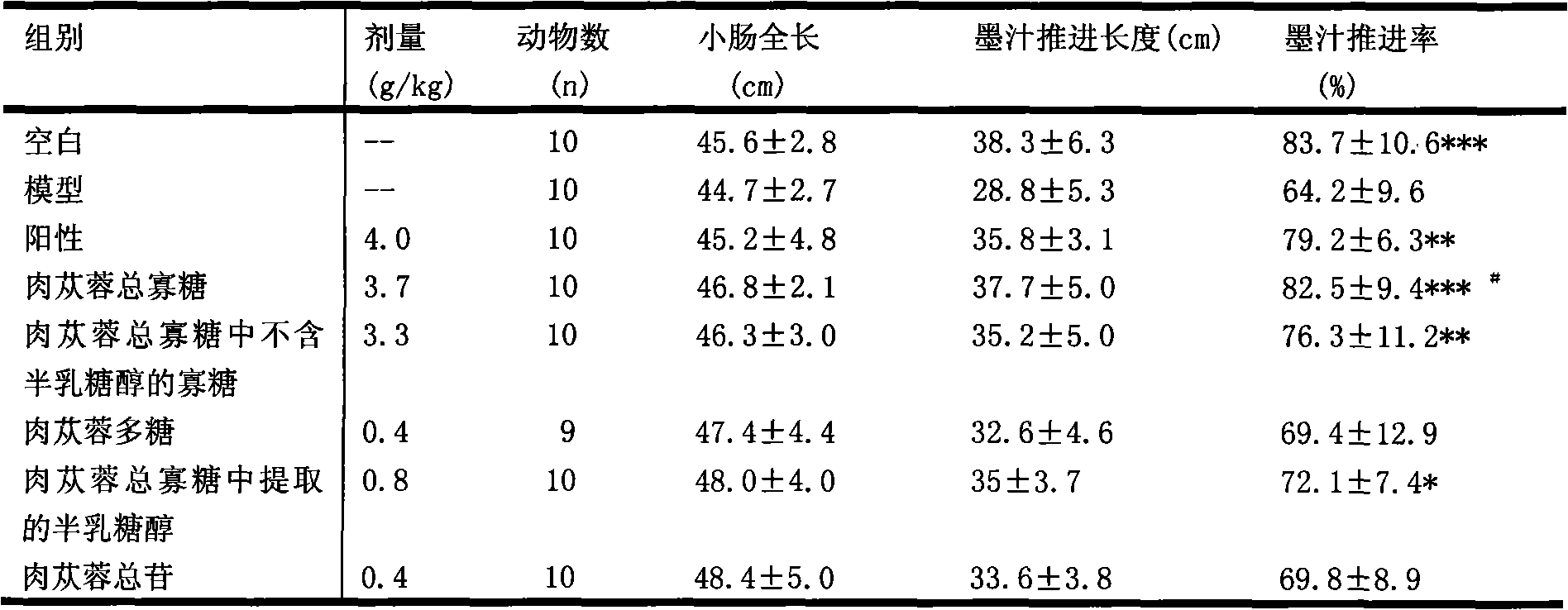
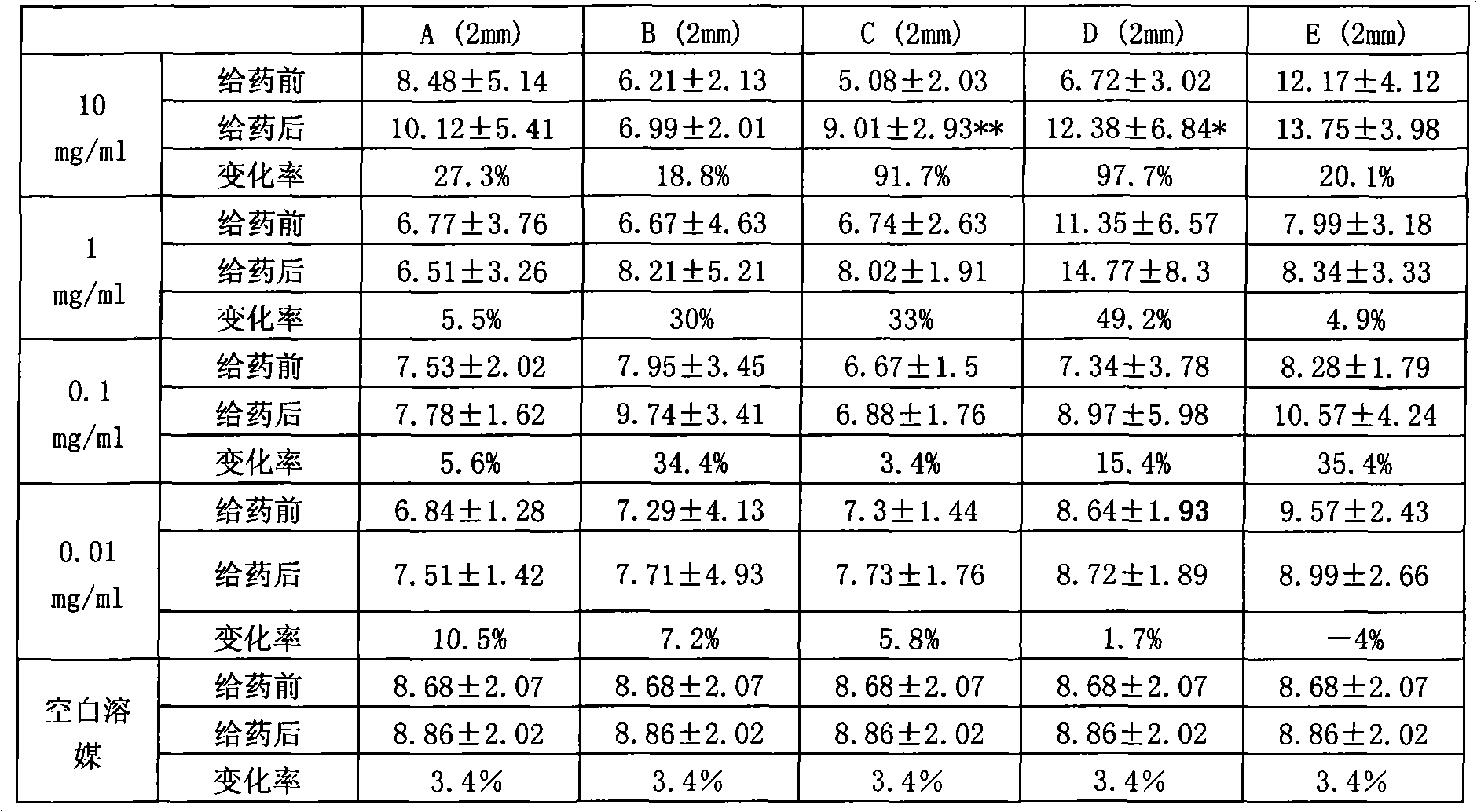
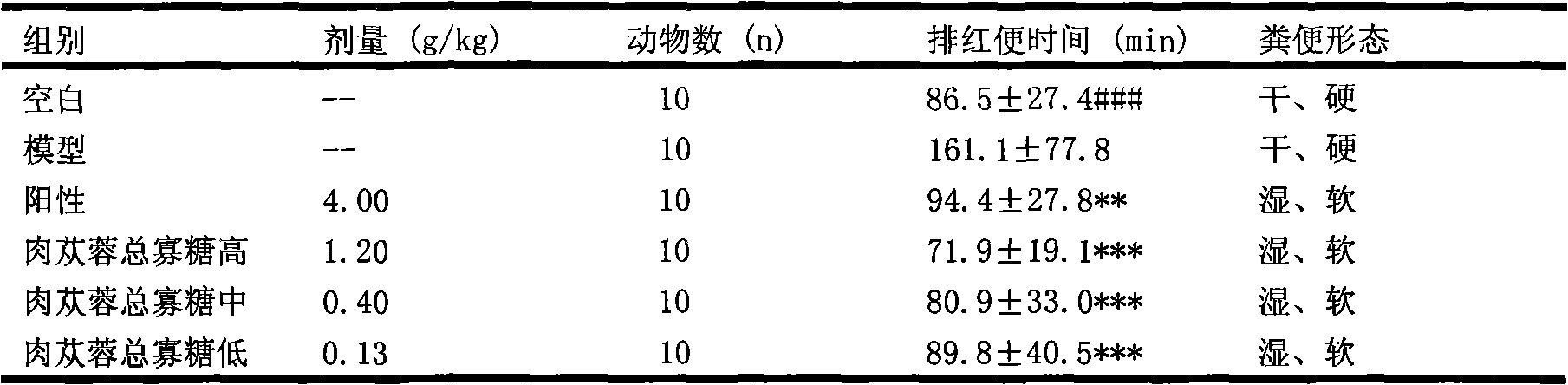
Examples
Embodiment 1
[0068] Take 100g of Herba Cistanche, cut into slices, add crude drug 1L of water, heat and reflux to extract for 1 hour, filter, and the filtrate is used for later use; Concentrate under pressure until the relative density is 1.20 at 50°C to obtain concentrated solution I; place the concentrated solution I at room temperature, slowly add 80% ethanol to make the alcohol content reach 70%, place it in a 6°C freezer for 24 hours, filter, and the filtrate reduces Concentrate under pressure to obtain Concentrate II; place Concentrate II at room temperature, slowly add to AB-8 macroporous adsorption resin column with resin column diameter-to-height ratio of 1:12, the ratio of medicinal material to dry resin is 1:1, and use 2 Times column volume of deionized water was eluted, and the water eluate was concentrated under reduced pressure to a thick paste to obtain 28.6 g of total oligosaccharides of Cistanche deserticola; 6 h 12 o 6 Not less than 58%, the total oligosaccharides conta...
Embodiment 2
[0070] Take 100g of Herba Cistanche, cut into slices, add crude drug 0.6L of water, heat and reflux for extraction for 3 hours, filter to obtain the filtrate, and set aside; add 0.4L of water to the dregs, extract 3 times, each time for 0.5 hours, filter, and combine four The second filtrate was concentrated under reduced pressure until the relative density was 1.10 at 70°C to obtain the concentrated solution I. Place the concentrated solution I at room temperature, slowly add 95% ethanol to make the alcohol content reach 50%, place it in a refrigerator at 2°C for 6 hours, filter When the filtrate was concentrated under reduced pressure to no alcohol, the relative density was 1.10, and the concentrated solution II was obtained. The concentrated solution II was placed at room temperature, and slowly added to the HPD-100 macroporous adsorption resin column with a resin column diameter-to-height ratio of 1:6. , the ratio of medicinal material to dry resin is 1:3, elute with 2 time...
Embodiment 3
[0072] Take 100g of Herba Cistanche, cut into slices, add crude drug 0.8L of water to extract under reflux for 2 hours, filter, and use the filtrate for later use; extract the dregs with 0.6L of water for 2 times, each time for 1 hour, and filter; combine the filtrate three times, Concentrate under reduced pressure to a relative density of 1.15 at 60°C to obtain concentrated solution I, place it at room temperature, slowly add 90% ethanol to make the alcohol content reach 60%, place it in a refrigerator at 4°C for 12 hours, filter, and concentrate the filtrate under reduced pressure. The concentrated solution II was obtained, placed at room temperature, and slowly added to a ME-2 macroporous adsorption resin column with a resin column diameter-to-height ratio of 1:9. The ratio of medicinal materials to dry resin was 1:2, and deionized water with 1.5 column volume Elution, collect the water eluate, concentrate under reduced pressure to a thick paste, and obtain 39.5 g of total o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com