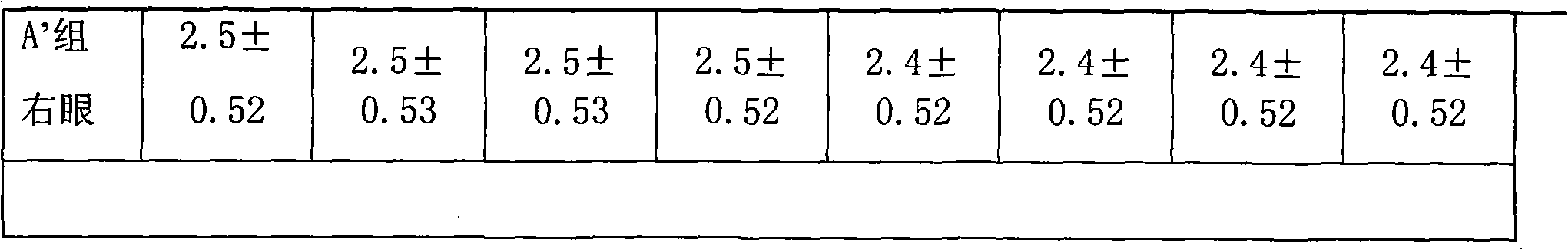Medicament composition and use thereof in preparing medicament for treating inflammation of eye section
A composition and drug technology, applied to the pharmaceutical composition of ocular inflammation, the field of treatment of ocular diseases, can solve the problems of combined use of prostaglandins, inability to obtain, etc., to overcome adverse reactions, overcome the increase in intraocular pressure, avoid The effect of elevated intraocular pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
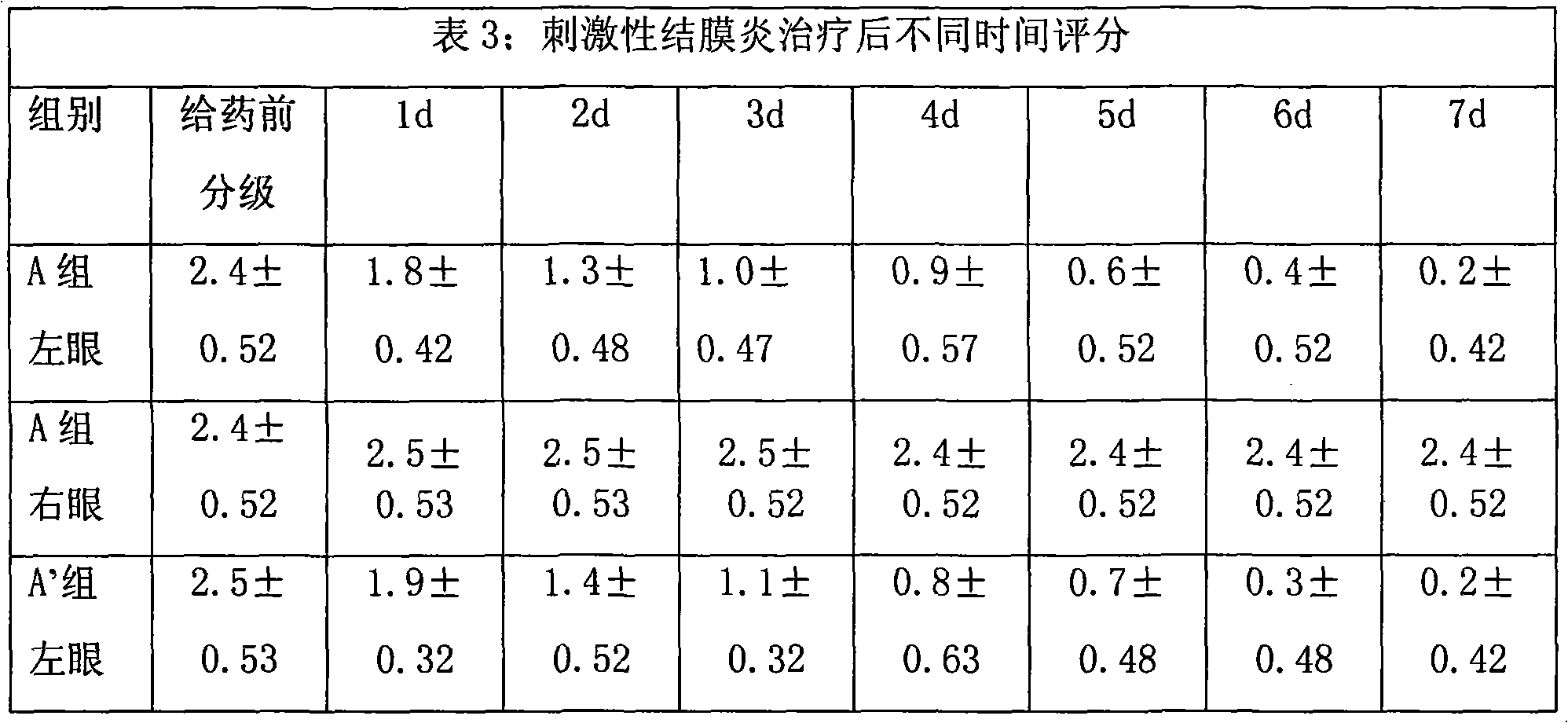
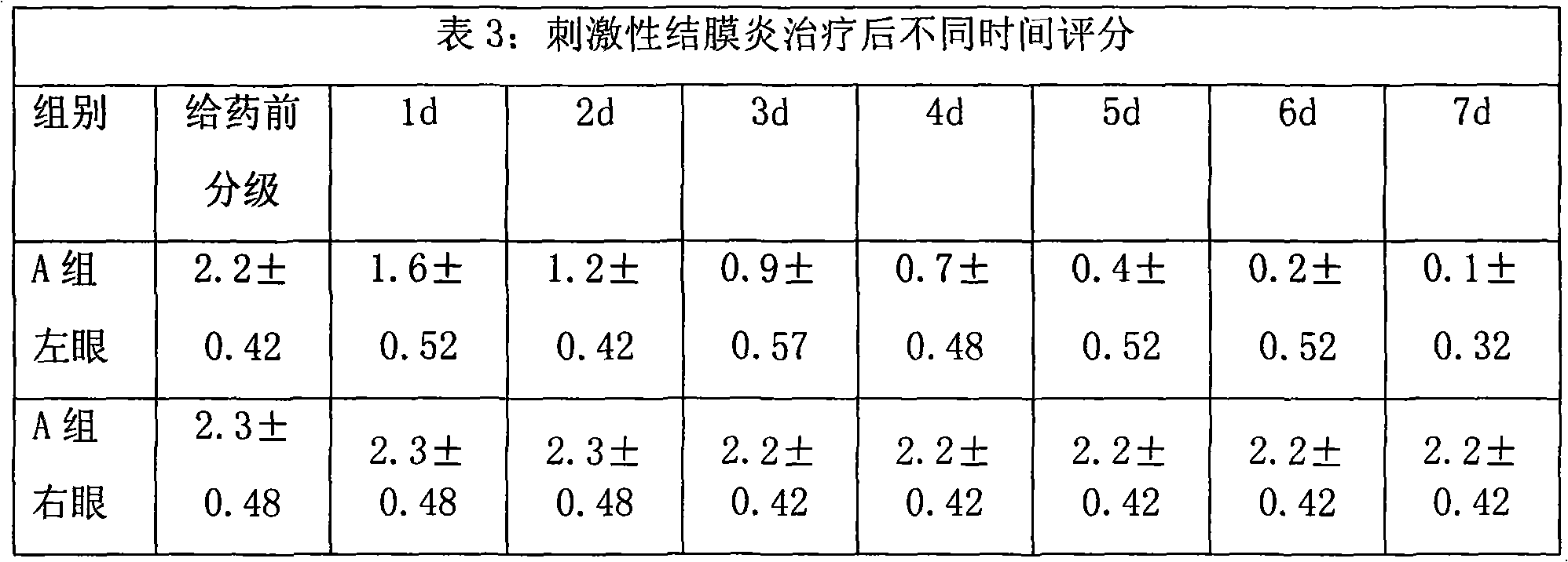
Image
Examples
Embodiment 1
[0041] Travoprost 10mg
[0042] Hydrocortisone acetate 1.0g (particle size 5-20μm)
[0043] Sodium Carboxymethyl Cellulose 2.0g
[0044] Tween-80 0.8g
[0045] Sodium dihydrogen phosphate 4.55g
[0046] Disodium hydrogen phosphate 6.55g
[0048] Ethylparaben 0.1g
[0049] Propylparaben 0.1g
[0050] Water for injection 1000ml
[0051] Dissolve the prescribed amount of ethylparaben and propylparaben in water for injection with 30% of the prescribed amount, heat to 80-90°C, add the prescribed amount of travoprost, Tween-80, dihydrogen phosphate Dissolve sodium, disodium hydrogen phosphate, and sodium chloride, filter with a No. 3 vertical melting funnel, and use it as solution, dissolve sodium carboxymethylcellulose in water for injection with 50% prescription volume, and use a pad with 200 Filter through a Buchner funnel of mesh nylon cloth, heat to 80-90°C, add the prescribed amount of hydrocortisone acetate and stir well, keep warm for 3...
Embodiment 2
[0053] Latanoprost 20mg
[0054] Hydrocortisone acetate 10g (particle size 5-20μm)
[0055] Sodium Carboxymethyl Cellulose 1.5g
[0056] Tween-80 1.0g
[0057] Sodium dihydrogen phosphate 7.9g
[0058] Sodium chloride 4.0g
[0059] Ethylparaben 0.15g
[0060] Propylparaben 0.15g
[0061] Water for injection 1000ml
[0062] Take 30% of the prescribed amount of water for injection, add the prescribed amount of sodium dihydrogen phosphate and sodium chloride to dissolve, adjust the pH to 6.5 with 1N sodium hydroxide solution, then add the prescribed amount of Tween-80 and paraben Ethyl ester, propylparaben, and latanoprost were filtered with No. 3 vertical melting funnel, and used as solution, sodium methylcellulose was dissolved in water for injection with 50% of the prescription volume, and a 200-mesh pad was used. Filter through a Buchner funnel of nylon cloth, heat to 80-90°C, add the prescribed amount of hydrocortisone acetate and stir well, keep warm for 30 minutes,...
Embodiment 3
[0064] Bimatoprost 100mg
[0065] Hydrocortisone acetate 10g (particle size 5-20μm)
[0066] Hydroxypropyl methylcellulose (non-ionic) 2.0g
[0067] Sodium dihydrogen phosphate 6.5g
[0068] Disodium hydrogen phosphate 5.2g
[0069] Sodium chloride 3.0g
[0070] 1N sodium hydroxide solution appropriate amount
[0071] Benzalkonium Chloride 0.25g
[0072] Dissolve the prescribed amount of benzalkonium chloride in 50% of the prescribed amount of water for injection, add the prescribed amount of sodium dihydrogen phosphate and sodium chloride to dissolve, adjust the pH to 6.8 with 1N sodium hydroxide solution, and then add the prescribed amount The amount of latanoprost was heated to 80-90°C to dissolve, cooled to room temperature, filtered with a No. 3 vertical melting funnel, and used as solution, hydroxypropyl methylcellulose was dissolved in 30% of the prescription amount for injection In water, filter with a Buchner funnel with 200-mesh nylon cloth, heat to 80-90°C, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com