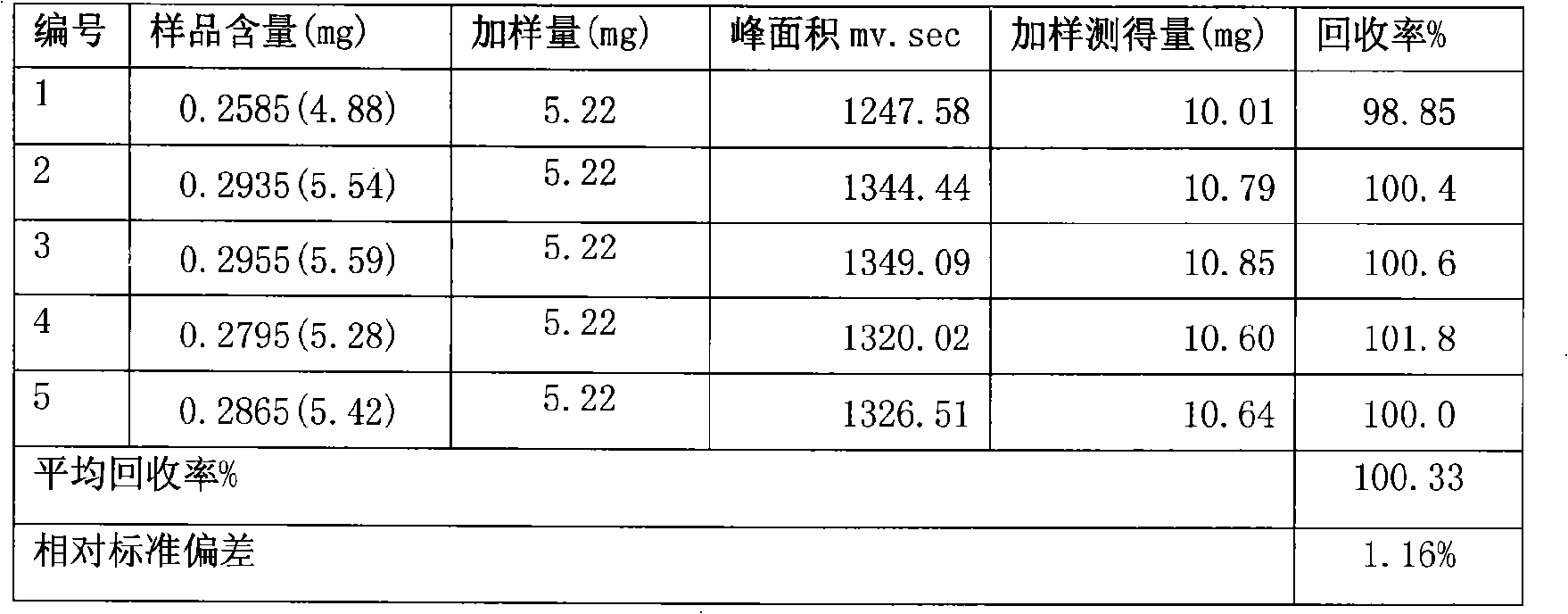
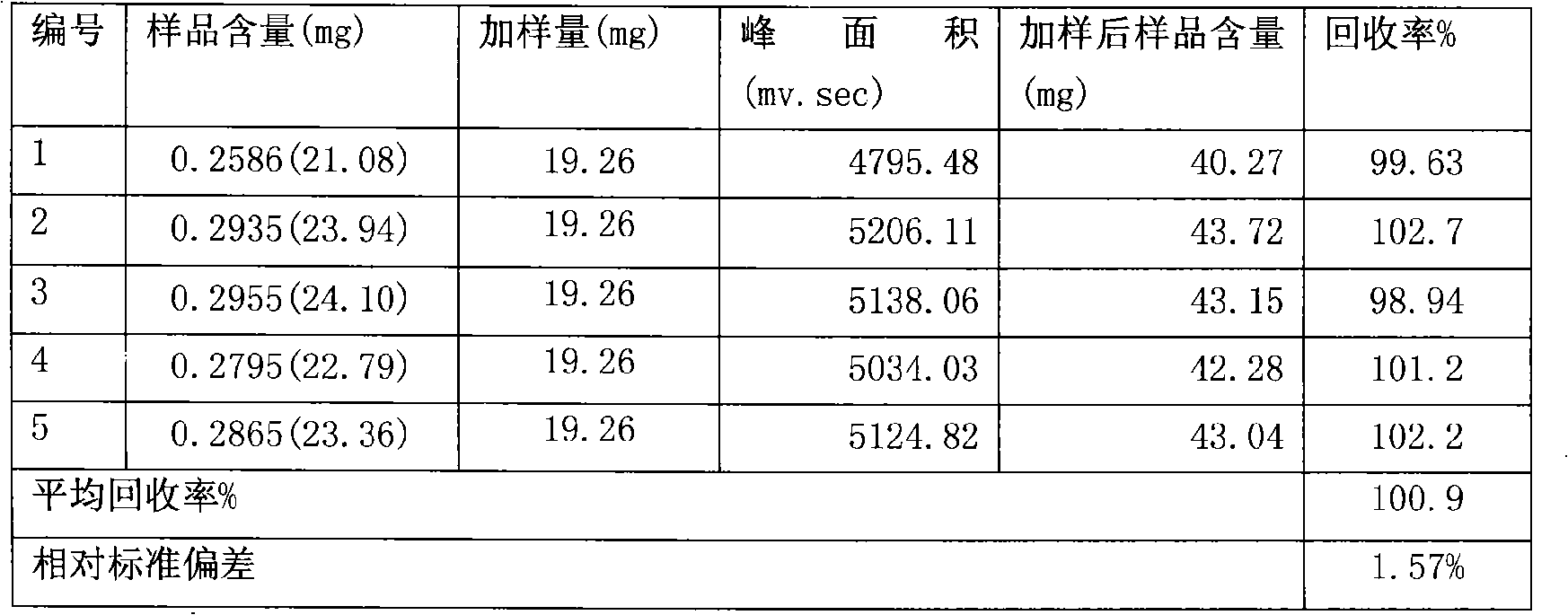
Tongbianling preparation and quality control method
A quality control method and laxative technology, which can be used in pill delivery, pharmaceutical formulations, measuring devices, etc., can solve problems such as long heating time, low extraction rate, thermal instability of active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 Granule (sugar-free type)
[0019] [Prescription] Senna 7200g Angelica 900g Cistanche 900g
[0020] [Preparation method] take senna leaf coarse powder (20 ~ 30 orders) according to the percolation method under the liquid extract and extract item (Chinese Pharmacopoeia 2005 edition one appendix I O), make solvent ultrasonic with three times amount of 50% ethanol After 30 minutes of treatment, carry out percolation, collect 8 times the amount of percolation liquid of medicinal materials, concentrate under reduced pressure at low temperature to a clear paste with a relative density of 1.05-1.15 (60°C), spray dry (inlet air temperature 150-170°C, temperature 100-105°C) to make extract powder; 450g of angelica was crushed into fine powder, and the remaining angelica and Cistanche were decocted twice with 8 times of water, each time for 1.5 hours, filtered, and the filtrate was concentrated to a relative density of 1.20-1.26 (80°C) extract; take senna leaf extra...
Embodiment 2
[0021] Example 2 tablet
[0022] [Prescription] Senna 1200g Angelica 150g Cistanche 150g
[0023] [Preparation method] get senna leaf coarse powder (20 order powders), according to the percolation method (Chinese Pharmacopoeia 2005 edition an appendix 10) under the liquid extract and extract item, make solvent ultrasonic with 30% ethanol three times amount After 30 minutes of treatment, carry out percolation, collect 8 times the percolation liquid of the medicinal material, concentrate under low temperature and reduced pressure to a ointment with a relative density of 1.16-1.22 (60°C), spray dry (inlet air temperature 160-170°C, outlet air temperature 50~60℃) into extract powder. 75g of angelica is crushed into fine powder, and the remaining angelica and cistanche are decocted twice with 8 times the amount of water, each time for 1.5 hours, filtered, and the filtrate is concentrated into an extract with a relative density of 1.20-1.26 (80°C); take senna leaves Extract powder...
Embodiment 3
[0024] Embodiment 3 capsules
[0025] [Prescription] Senna 1200g Angelica 150g Cistanche 150g
[0026] [Preparation method] get senna leaf coarse powder (20 mesh powder), according to the percolation method (Chinese Pharmacopoeia 2005 edition an appendix I0) under the liquid extract and the extract item, add three times the amount of water as the solvent ultrasonic treatment 30 Carry out percolation after 10 minutes, collect the percolation liquid of 8 times the amount of medicinal material, concentrate under low temperature and reduced pressure to a ointment with a relative density of 1.16~1.22 (60°C), spray dry (inlet air temperature 160~170°C, air outlet temperature 50~ 60°C) into extract powder. 75g of angelica is crushed into fine powder, and the remaining angelica and cistanche are decocted twice with 8 times the amount of water, each time for 1.5 hours, filtered, and the filtrate is concentrated into an extract with a relative density of 1.20-1.26 (80°C); take senna le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com