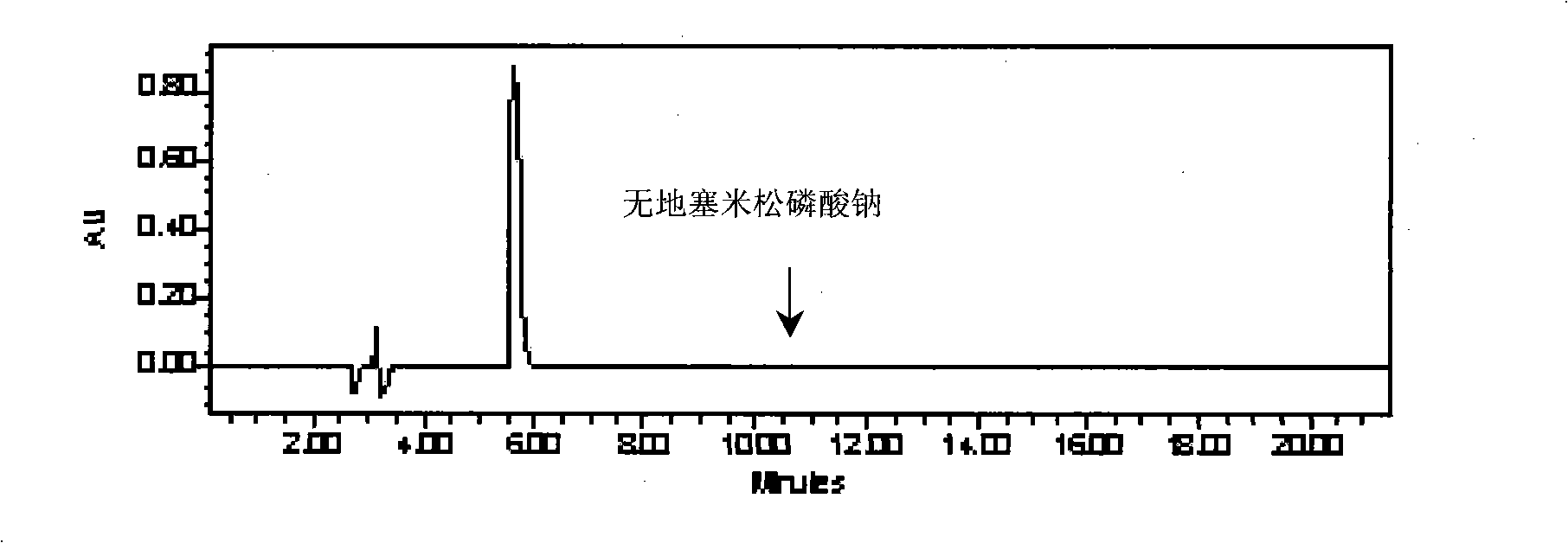
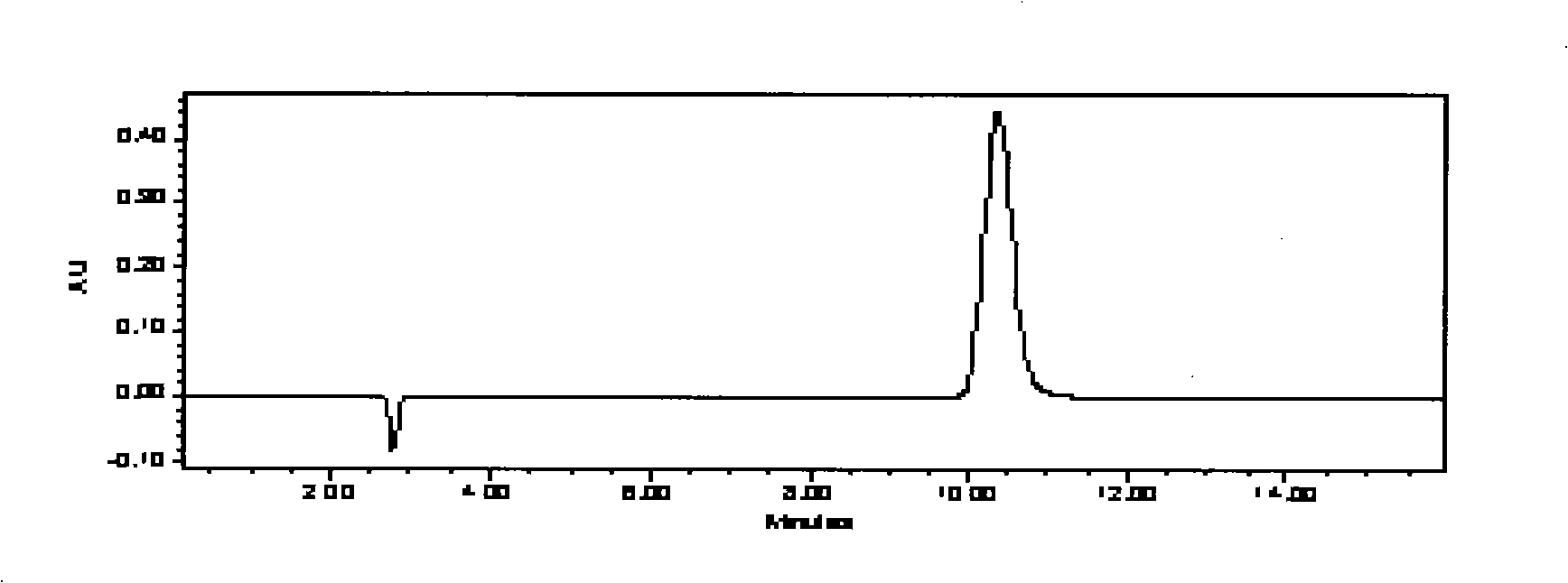
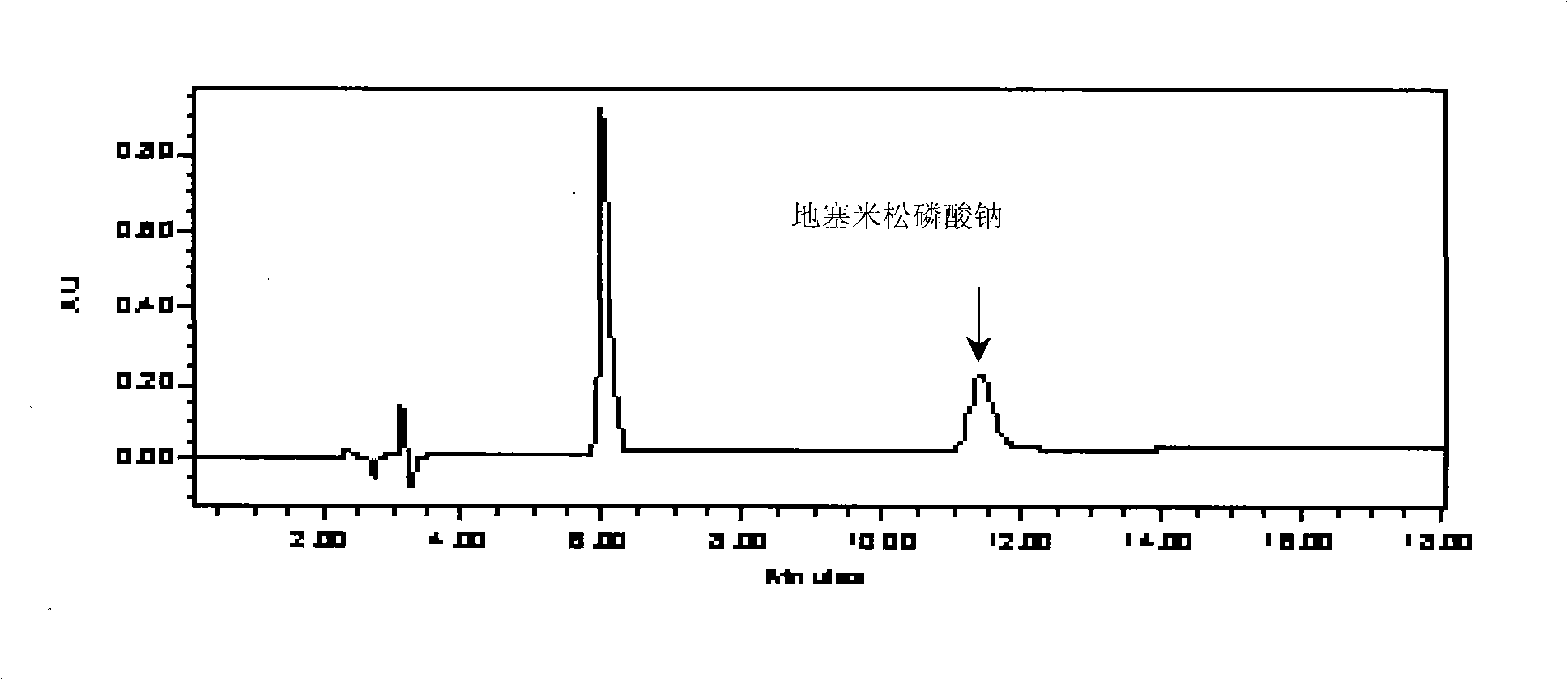
Formula for preparing anti-eczema and cutitis products and preparation method thereof
A technology for eczema dermatitis and formula, which is applied to the formulation and preparation field of anti-eczema dermatitis products, can solve problems such as diagnosis, detection, prevention and protection of eczema dermatitis that have not yet been found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0452] Embodiment 1, the preparation of this anti-eczema dermatitis product
[0453] The preparation of preferred scheme 6
[0454] 1 Sources and requirements of raw and auxiliary materials:
[0455] Phenol: Manufactured by Tianjin Bodi Chemical Co., Ltd., in line with the standards of Part II of the 2005 edition of the Chinese Pharmacopoeia (page 325). This product is a colorless to red needle-like crystal or crystalline block; has a special odor; has hygroscopicity; the aqueous solution shows a weak acid reaction; the color gradually becomes darker when exposed to light or in the air. The freezing point of this product is not lower than 40°C.
[0456] Menthol: Manufactured by Nantong Mint Factory Co., Ltd., in line with the standard of Part One (284 pages) of the 2005 edition of the Chinese Pharmacopoeia. This product is colorless acicular or prismatic crystal or white crystalline powder; it has the special aroma of mint, and the taste is hot at first and then cool. Etha...
Embodiment 2
[0472] Embodiment 2, dermatitis tincture (preferred scheme 4) clinical application summary
[0473] Abstract OBJECTIVE: To study the safety, feasibility and effect of dermatitis tincture (formula 4) in the clinical treatment of pruritus such as eczema and contact dermatitis. Method: 60 outpatients were selected and randomly divided into test group and control group, 60 cases in the test group and 40 cases in the control group. In the test group, dermatitis tincture was sprayed on the affected area, and cool oil was used as control, 3 to 4 times a day. Changes in skin lesions and itching sensation were observed within one month. Results: Compared with the qingliangyou control group, the total effective rate of eczema, contact dermatitis, skin amyloidosis and chronic prurigo was significantly different (P<0.05). Discussion: This dermatitis tincture has the advantages of convenient use, quick effect, and mild side effects. It is effective and safe for the treatment of eczema, co...
Embodiment 3
[0495] Embodiment 3, dermatitis tincture (preferred scheme 5) clinical application summary
[0496] Abstract OBJECTIVE: To study the safety, feasibility and effect of dermatitis tincture (formulation 5) in the clinical treatment of neurodermatitis, solar dermatitis and atopic dermatitis. Methods: 60 outpatients were selected and randomly divided into test group and control group, 40 cases in the test group and 20 cases in the control group. In the test group, dermatitis tincture was sprayed on the affected area, and cool oil was used as control, 3 to 4 times a day. Changes in skin lesions and itching sensation were observed within one month. Results: Compared with the qingliangyou control group, there were significant differences in the total effective rate of neurodermatitis, solar dermatitis and atopic dermatitis (P<0.05). Discussion: The present invention has the advantages of convenient use, quick effect, safety and no allergy, and is suitable for the treatment of neurode...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| dew point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com