Optimizing mass spectrogram model for detecting kidney cancer characteristic protein and preparation method and application thereof
A technology of characteristic protein and mass spectrometry model, applied in the field of mass spectrometry detection and protein detection, can solve the problems of inability to detect low-abundance proteins, insufficient resolution, limited practical value, etc., to improve clinical cure, reduce fatality rate, and design accurate and reasonably practicable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Discrimination of normal and renal cancer patients and preparation of mass spectrometry kit
[0051] (1) Experimental method
[0052] 64 cases of renal cell carcinoma patients (including 14 cases of stage I, 29 cases of stage II, 14 cases of stage III, 7 cases of stage IV, aged 32 to 77 years, median age of 48 years) and 26 patients with benign renal disease (age 36 years old) ~71 years, median age 45 years) of preoperative serum. 90 control sera were from healthy volunteers (aged 25-69 years old, median age 45 years old), from the physical examination population with normal liver function and renal function. 1 mL of venous blood was collected from the subjects on an empty stomach. Immediately after collection, they were placed in a refrigerator at 4 °C for 2 hours, centrifuged at 4000 r / min at 4 °C for 10 minutes to separate the serum, and the serum was centrifuged again at 12000 r / min at 4 °C for 5 minutes to remove all residual cell debris. and insoluble ...
Embodiment 2
[0078] Example 2 Clinical trial and double-blind test
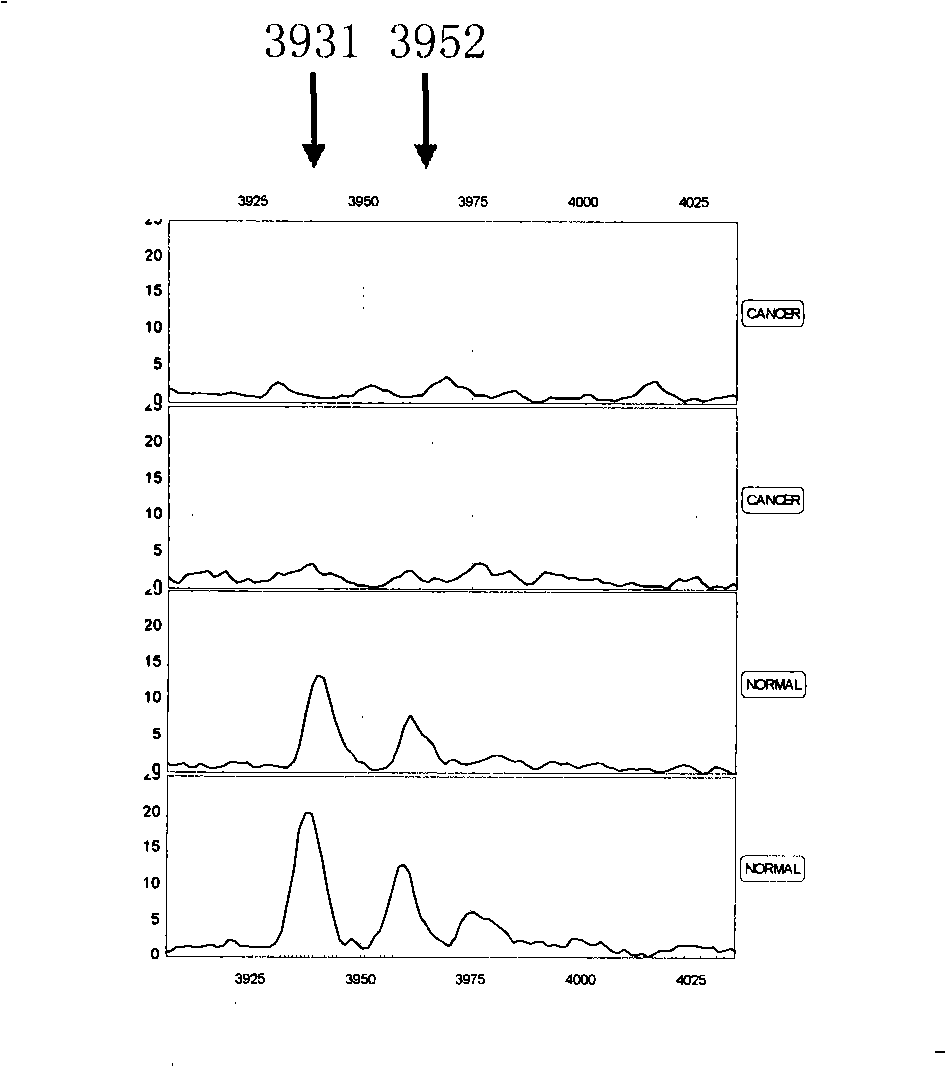
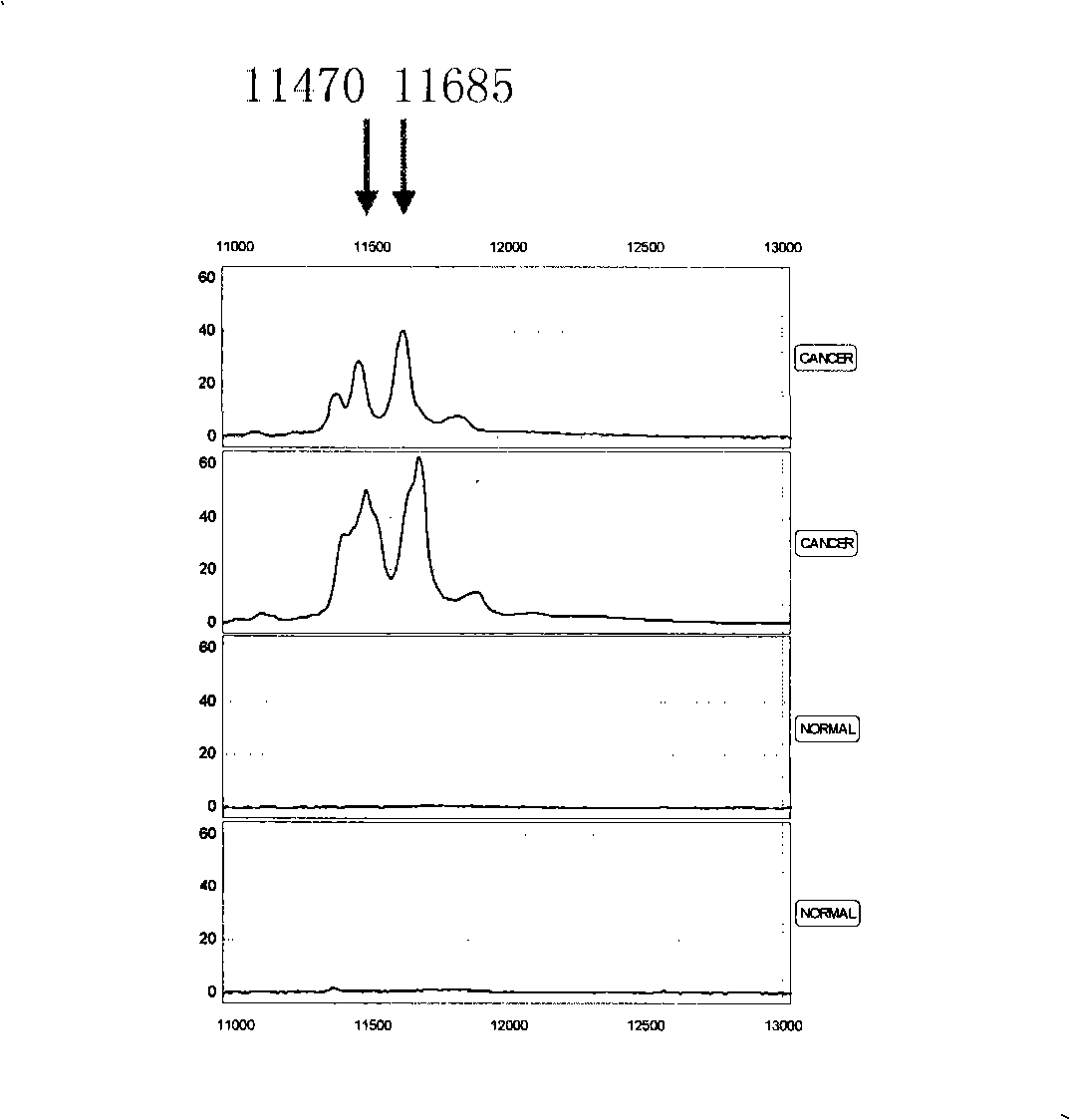
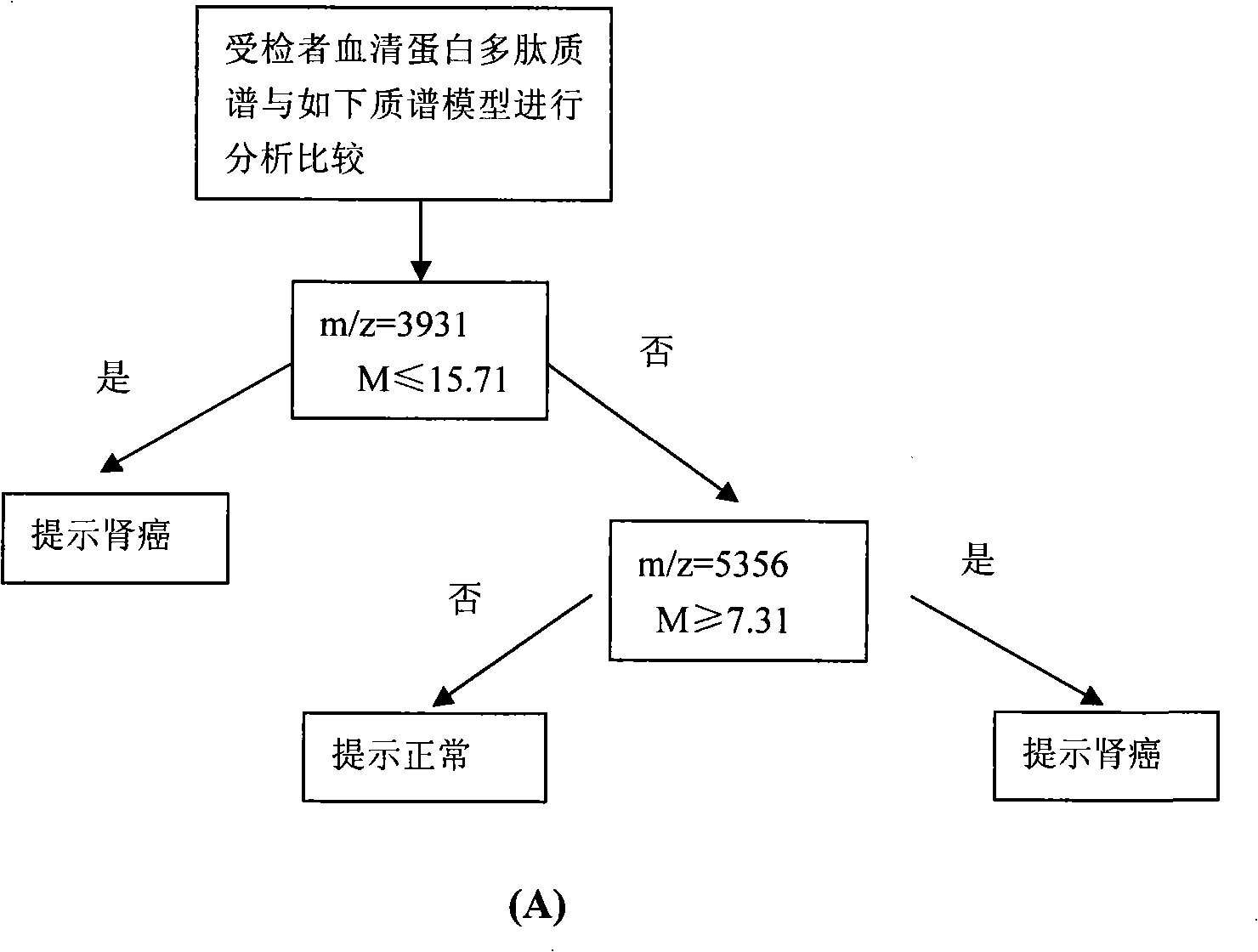
[0079] Since the combination of multiple characteristic proteins can completely separate renal cancer from normal people, any two or more of the above 10 characteristic proteins are selected, and the m / z value of each protein peak is calculated according to the mass-to-charge ratio of each protein peak. And based on the critical peak mean value M of this protein, a mass spectrometry model of serum characteristic protein detection was established for the identification of renal cancer patients and normal people, benign renal disease, renal cancer lymph node metastasis and renal cancer distant metastasis patients ( Figure 3-5 ), the specific protein mass-to-charge ratio m / z and the critical peak mean value M are respectively m / z=3931, M≤15.71; m / z=5356, M≥7.31; m / z=5474, M≤4.83; m / z=3935, M≤7.76; m / z=3190, M≥7.21; m / z=4021, M≥3.29; m / z=11685, M≥22.51; m / z=15076, M≥20.71; Among them, the mass spectrometry model A for distin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com