Fructo oligosaccharide maleate and method for preparing the same
A technology of fructooligosaccharides and maleate, which is applied in oligosaccharides, food preparation, food science and other directions, can solve the problems of not being able to heat for a long time, limiting the scope of use of fructooligosaccharides, etc., and achieves good acid resistance and heat resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Weigh 20g of maleic anhydride, put the maleic anhydride in a 250mL Erlenmeyer flask, cover it and put it on an electric furnace for 10 minutes to melt it, after melting, add fructooligosaccharide in several times in a water bath at 90°C, adding 5g in total fructooligosaccharides. After all fructo-oligosaccharides are dissolved, react in a water bath at 90°C for about 16 hours. After cooling to room temperature the result was a yellow solid. After washing and purifying with 80% ethanol and absolute ethanol, and vacuum drying at 60°C, the obtained fructooligosaccharide maleate is obtained. The product is light yellow powdery solid with sweet taste.
Embodiment 2
[0041] Weigh 30g of maleic anhydride, put the maleic anhydride in a 250mL Erlenmeyer flask, cover it and put it on the electric furnace for 10 minutes to melt, after melting, add fructooligosaccharides in several times in a water bath at 85°C, each time a small amount, A total of 5g fructooligosaccharides were added and shaken until dissolved. After all fructo-oligosaccharides are dissolved, react in a water bath at 90°C for about 20 hours. After cooling to room temperature the result was a yellow solid. After washing and purifying with 80% ethanol and absolute ethanol, and vacuum drying at 60°C, the obtained fructooligosaccharide maleate is obtained. The product is light yellow powdery solid with sweet taste.
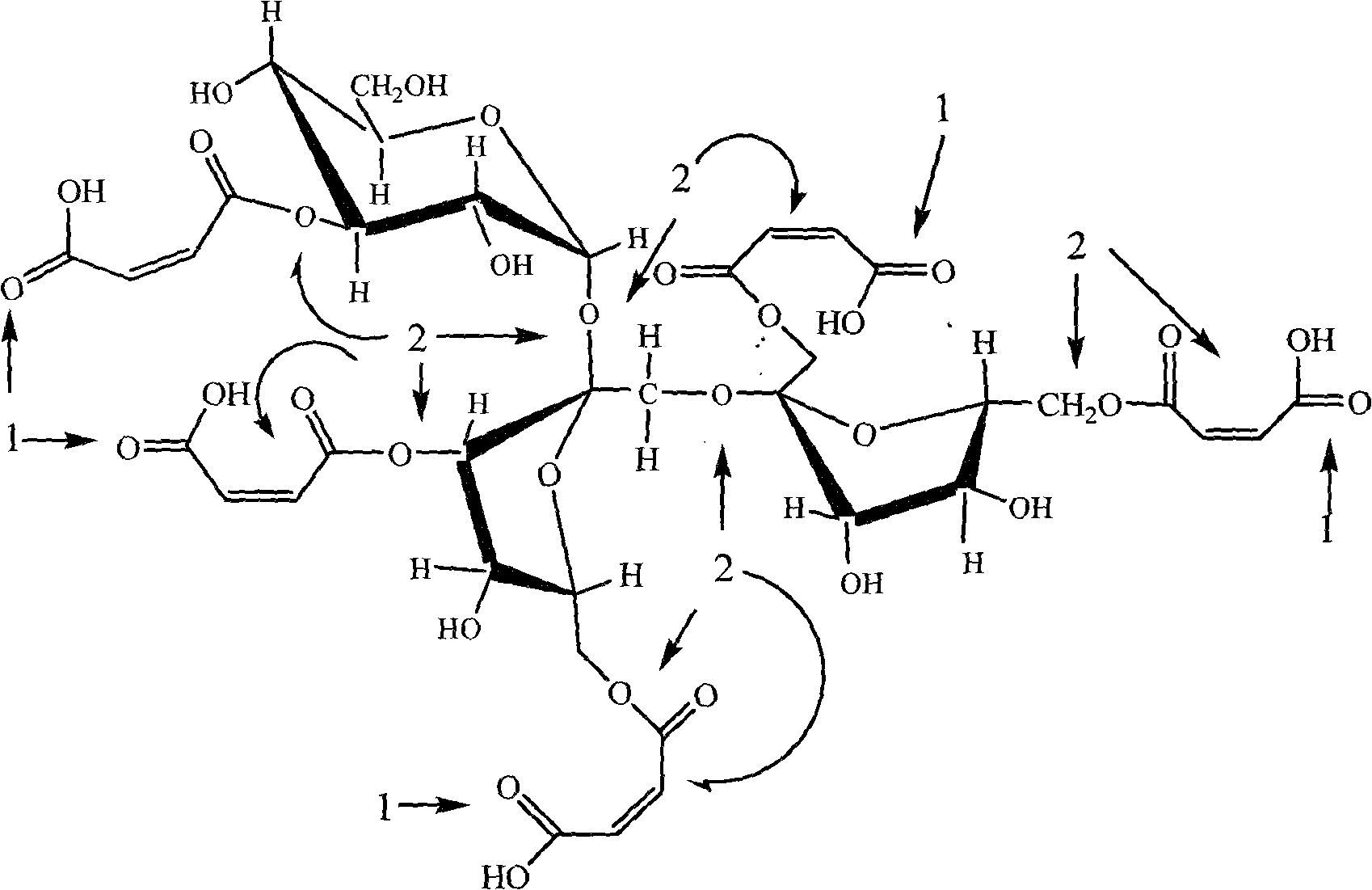
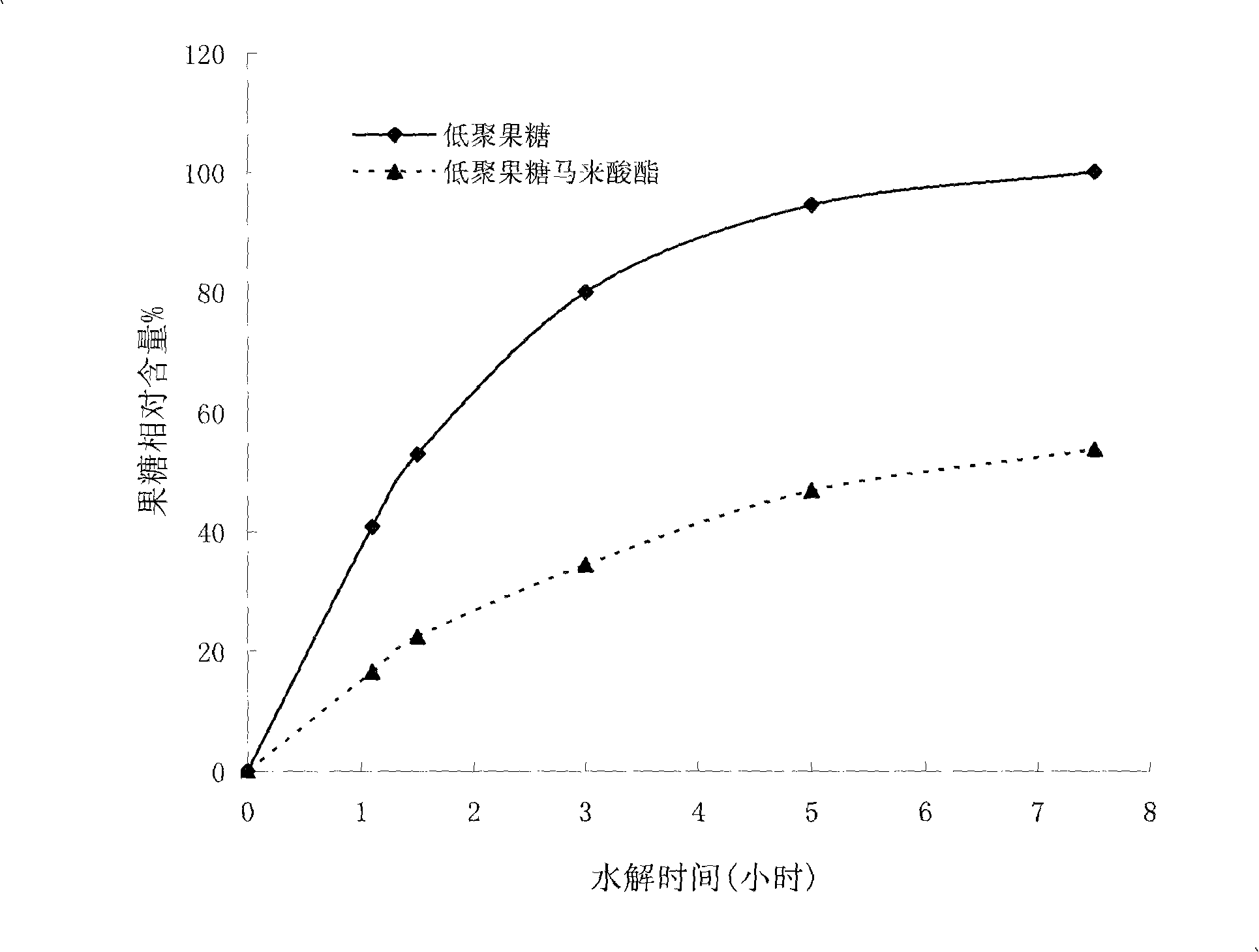
[0042] The structural representation of fructooligosaccharide maleate of the present invention is as figure 1 shown, combined with figure 2 , it not only has the sweetness and functionality of fructooligosaccharide, but also has better acid and heat resistance tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com