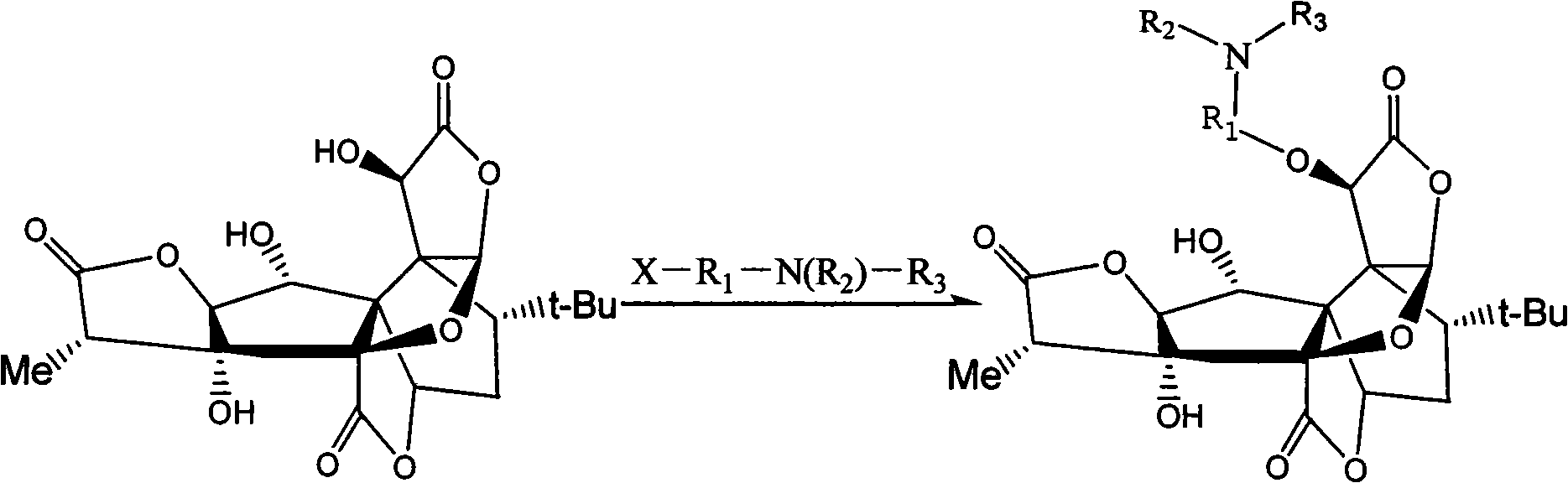Synthetic technological process of bilobalide B derivates
A technology of ginkgolide and synthesis process, which is applied in the field of medicine, can solve problems such as retention, actual production operation and hidden safety hazards, and achieve the effects of cost simplification, cost reduction, and increased reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
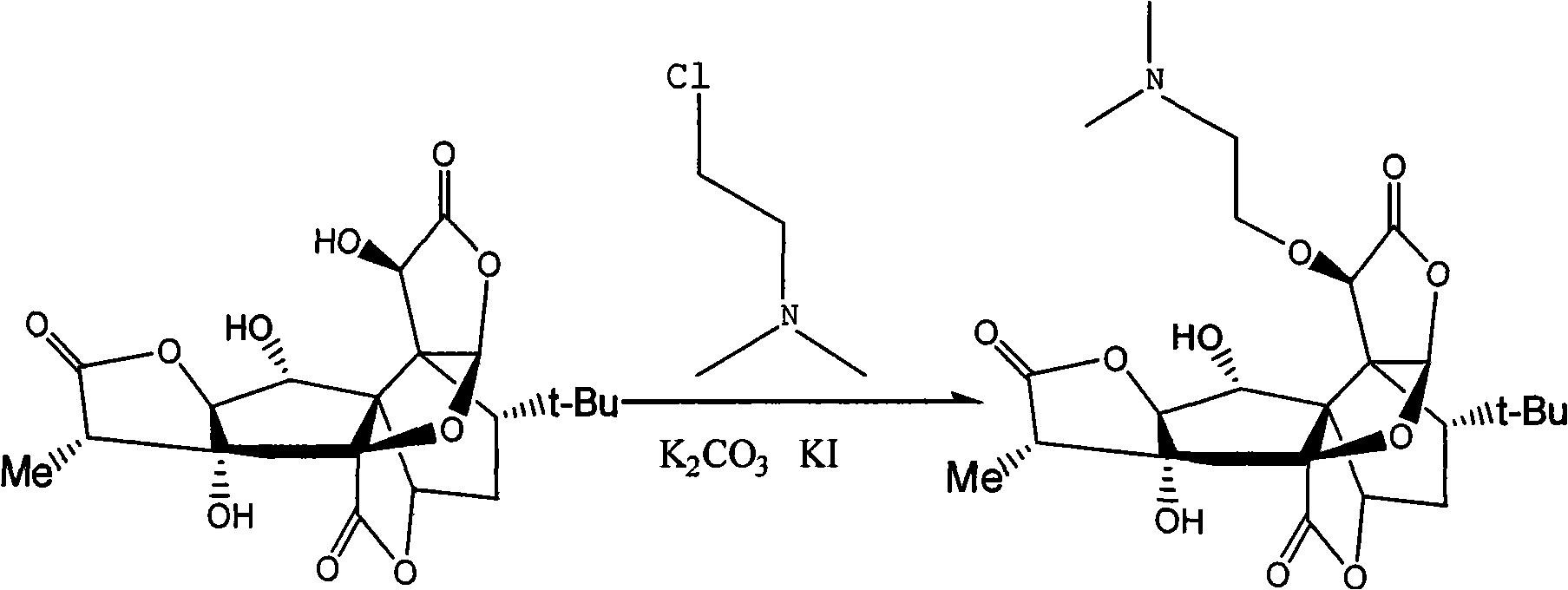
[0014] Example 1 Preparation of 10-O-(dimethylaminoethyl) ginkgolide B
[0015] 250g (0.59mol) of ginkgolide B was dissolved in 8.6L of acetonitrile, followed by adding 130g of dimethylaminoethyl chloride hydrochloride, 800g of potassium carbonate, 100g of potassium iodide, and 20g of tetrabutylammonium bromide, and heated to reflux for 1 hour. The reaction is almost complete. Cool, filter, and concentrate the filtrate under reduced pressure to obtain a crude solid. The crude product was purified, recrystallized from methanol, and dried to obtain 117 g of solid (yield 40%).
[0016] Reaction route:
[0017]
Embodiment 2
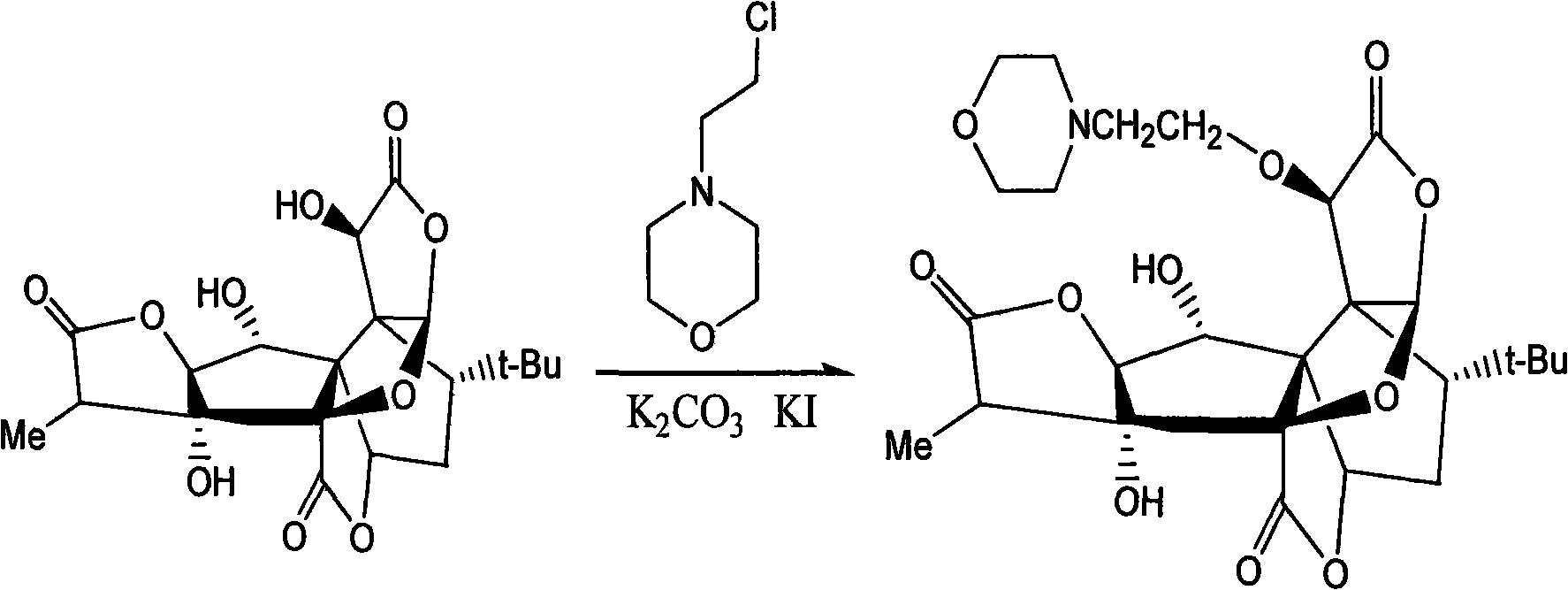
[0018] Example 2 Preparation of 10-O-(N-ethylmorpholide) ginkgolide B
[0019] 60g (0.14mol) of ginkgolide B was dissolved in 4L of acetonitrile, followed by adding 37g of N-(2-chloroethyl)morpholine hydrochloride, 250g of potassium carbonate, 25g of potassium iodide, cetyltrimethylammonium bromide 20g, heated to reflux until the reaction is almost complete. Cool, filter, and concentrate the filtrate under reduced pressure to obtain a crude solid. The crude product was purified, recrystallized from methanol, and dried to obtain 37 g of solid (yield 49%).
[0020] Reaction route:
[0021]
Embodiment 3
[0022] Example 3 Preparation of 10-O-(diethylaminoethyl) ginkgolide B
[0023] 250g (0.59mol) of ginkgolide B was dissolved in 8.6L of tetrahydrofuran, followed by adding 155g of diethylamino ethyl chloride hydrochloride, 800g of potassium carbonate, 100g of potassium iodide, and 18g of triethylbenzyl ammonium chloride, and heated to reflux for 1 Hours, the reaction was almost complete. Cool, filter, and concentrate the filtrate under reduced pressure to obtain a crude solid. The crude product was purified, recrystallized from methanol, and dried to obtain 125 g of solid (yield 41%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com