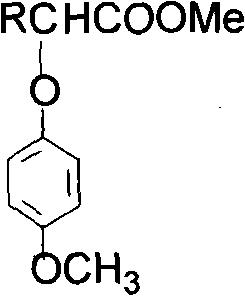
Synthetic method of 4-methoxyl phenoxyl alkylphenate
A kind of technology of methoxyphenoxyalkanoate and methoxyphenoxyalkanoic acid, which is applied in the field of synthesis of 4-methoxyphenoxyalkanoate, and can solve the problem of no artificial synthesis method. Patents and research reports, resource constraints, complex processes, etc., to achieve the effect of simple and fast synthesis method, low cost, and few side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
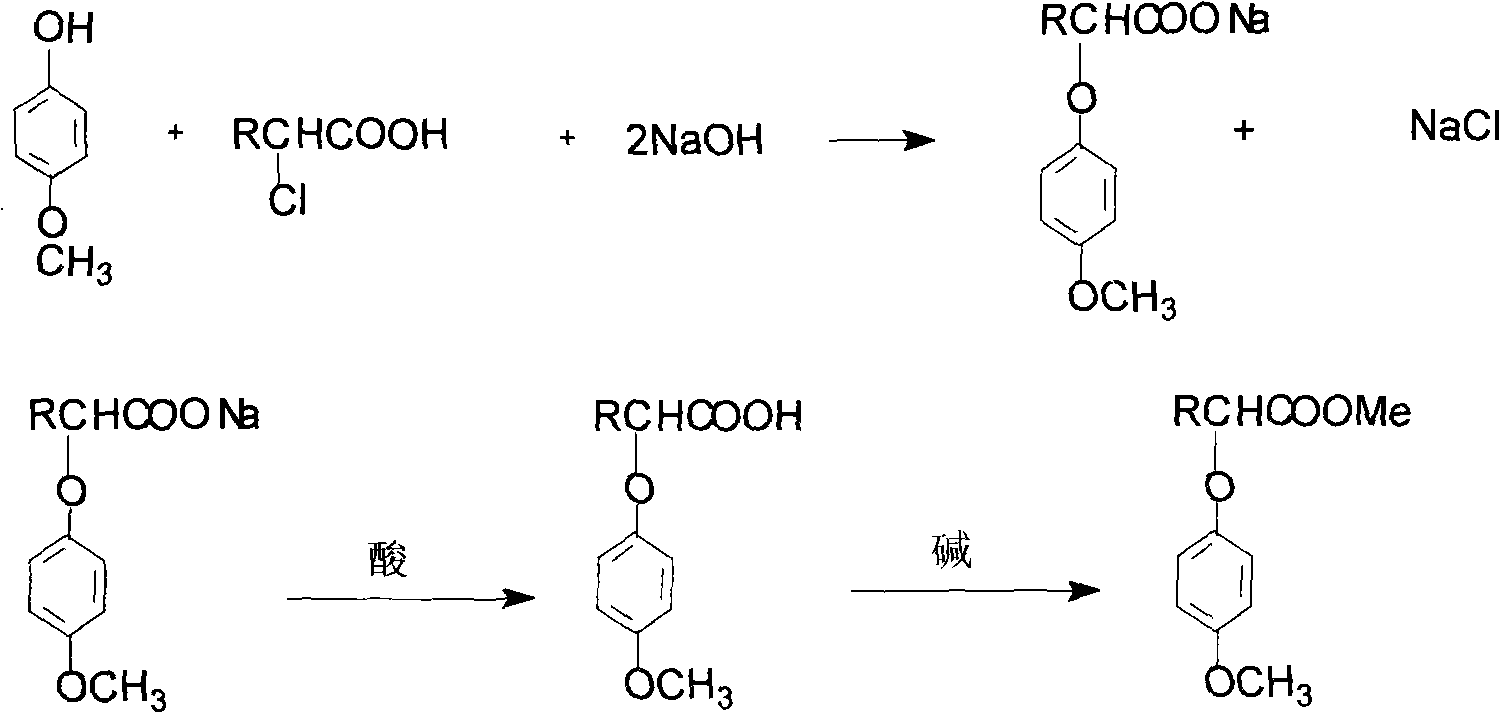
[0011] Example 1: 42 g of 4-methoxyphenol, 38 g of 2-chloropropionic acid, and 30 g of sodium hydroxide were added to a reactor, and the temperature was raised under stirring. At 80-120°C, with a pressure of 0-0.05MPa, react for 2 hours. After the reaction, it was acidified with sulfuric acid to generate 2-(4-methoxyphenoxy)propionic acid crystals. The crystals are neutralized with sodium carbonate to generate 2-(4-methoxyphenoxy)propionic acid sodium salt.
Embodiment 2
[0012] Example 2: 35 g of 4-methoxyphenol, 34 g of 2-chloropropionic acid, and 26 g of sodium hydroxide were added to the reactor, and the temperature was raised under stirring. At 100-130°C, the pressure is 0-0.1MPa, and react for 1 hour. After the reaction, it was acidified with sulfuric acid to generate 2-(4-methoxyphenoxy)propionic acid crystals. The crystals are neutralized with calcium hydroxide to generate calcium salt of 2-(4-methoxyphenoxy)propionate.
Embodiment 3
[0013] Example 3: 24 g of 4-methoxyphenol, 24 g of 2-chloropropionic acid, and 18 g of sodium hydroxide were added to the reactor, and the temperature was raised under stirring. At 70-110°C, with a pressure of 0-0.05MPa, react for 3 hours. After the reaction, it was acidified with sulfuric acid to generate 2-(4-methoxyphenoxy)propionic acid crystals. The crystals are neutralized with sodium hydroxide to generate 2-(4-methoxyphenoxy)propionic acid sodium salt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com