Synthetic method of 4-methoxyl phenoxyl alkylphenate
A technology of methoxyphenoxy alkyl salt and synthesis method, which is applied in the field of synthesis of food additives, can solve the problems of patents and research reports of no artificial synthesis method, resource constraints, complex process, etc., to achieve synthesis The method is simple and fast, with low cost and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
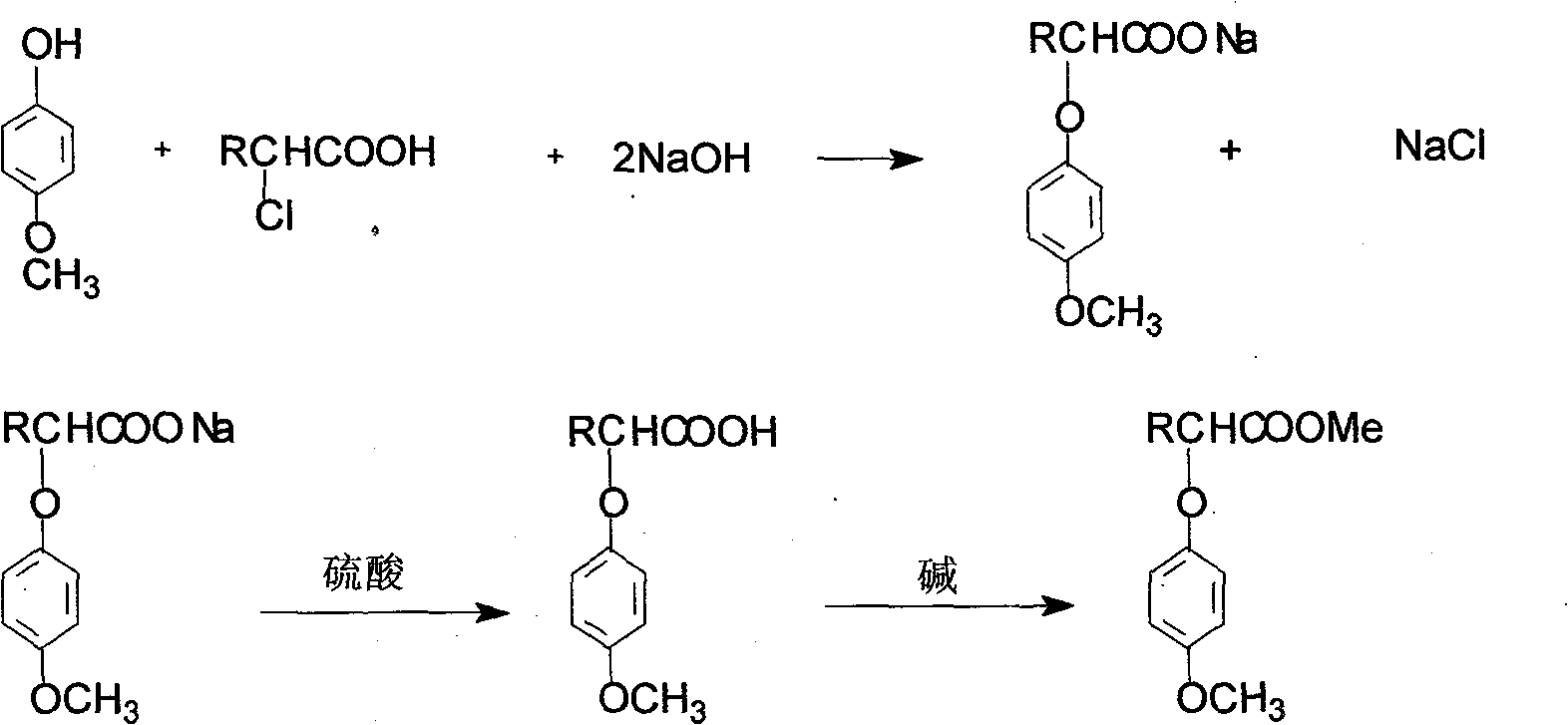
[0011] Example 1: Add 42g of 4-methoxyphenol, 38g of 2-chloropropionic acid, and 30g of sodium hydroxide into a reactor, and heat up under stirring. At 80-120°C, with a pressure of 0-0.05MPa, react for 2 hours. After the reaction, it was acidified with sulfuric acid to generate 2-(4-methoxyphenoxy)propionic acid crystals. The crystals are neutralized with sodium carbonate to generate 2-(4-methoxyphenoxy)propionic acid sodium salt.
example 2
[0012] Example 2: 35 g of 4-methoxyphenol, 34 g of 2-chloropropionic acid, and 26 g of sodium hydroxide were added to the reactor, and the temperature was raised under stirring. At 100-130°C, the pressure is 0-0.1MPa, and react for 1 hour. After the reaction, it was acidified with sulfuric acid to generate 2-(4-methoxyphenoxy)propionic acid crystals. The crystals are neutralized with calcium hydroxide to generate calcium salt of 2-(4-methoxyphenoxy)propionate.
example 3
[0013] Example 3: 24 g of 4-methoxyphenol, 24 g of 2-chloropropionic acid, and 18 g of sodium hydroxide were added to the reactor, and the temperature was raised under stirring. At 70-110°C, with a pressure of 0-0.05MPa, react for 3 hours. After the reaction, it was acidified with sulfuric acid to generate 2-(4-methoxyphenoxy)propionic acid crystals. The crystals are neutralized with sodium hydroxide to generate 2-(4-methoxyphenoxy)propionic acid sodium salt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com