Preparing methods and uses of S-(-)-nadifloxacin and water soluble salt thereof
A technology of nafloxacin and water-soluble salt, which is applied in the field of preparation of S--nafloxacin water-soluble salt, can solve the problem of poor effect of racemate nafloxacin, and achieve low gastrointestinal side effects , stable yield, and reduced toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
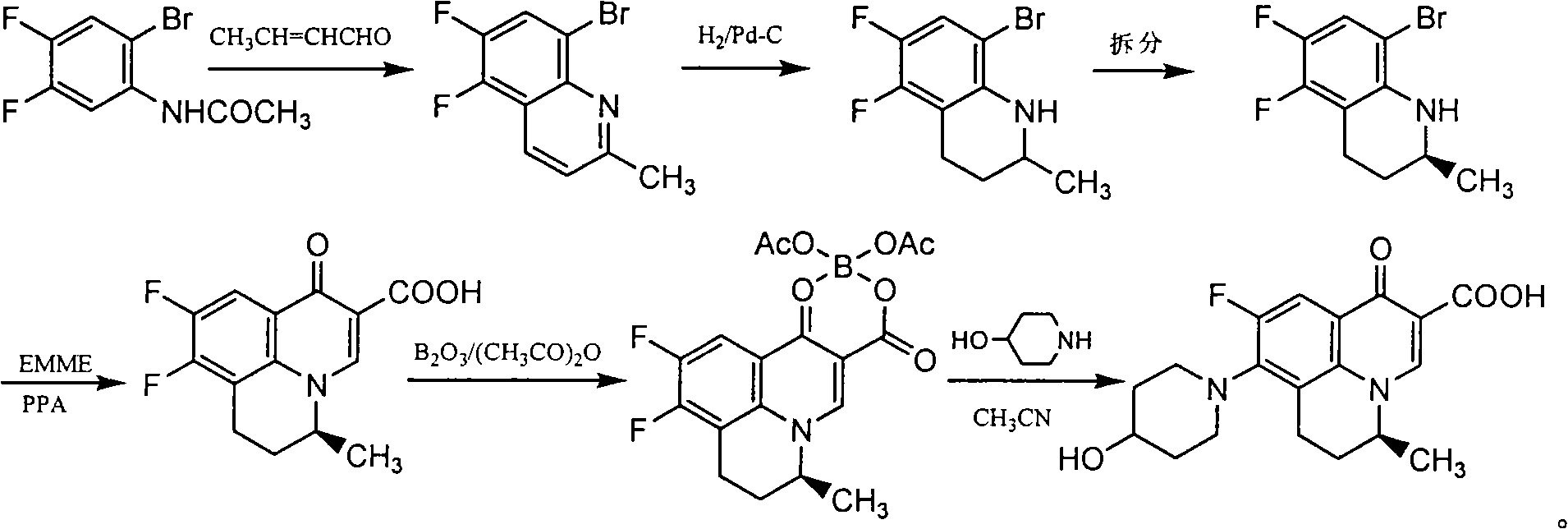
[0033] Embodiment 1: the preparation of (±)-5,6-difluoro-2-methyl-1,2,3,4-tetrahydroquinoline
[0034] Step 1: Preparation of 8-bromo-5,6-difluoro-2-methylquinoline
[0035]
[0036] Add 3,4-difluoro-6-bromophenylacetamide (160g, 0.64mol), sodium m-nitrobenzenesulfonate (144g, 0.64mol) into a three-necked flask equipped with stirring, condenser and dropping funnel , ferric sulfate heptahydrate (18g, 0.03mol), boric acid (174g, 2.81mol), water (560ml) and concentrated hydrochloric acid (560ml), heated to reflux under stirring conditions for 0.5 hour, slowly added dropwise crotonaldehyde at the same temperature (79ml, 0.97mol), the dropwise addition was completed in about 1 hour, and then refluxed for 1 hour, suction filtered while it was hot, the filtrate was cooled to room temperature, methanol (2300ml) was added, and then 25% sodium hydroxide solution was used to Adjust the pH value to about 10 to completely precipitate crystals, filter with suction, and wash twice with 5...
Embodiment 2
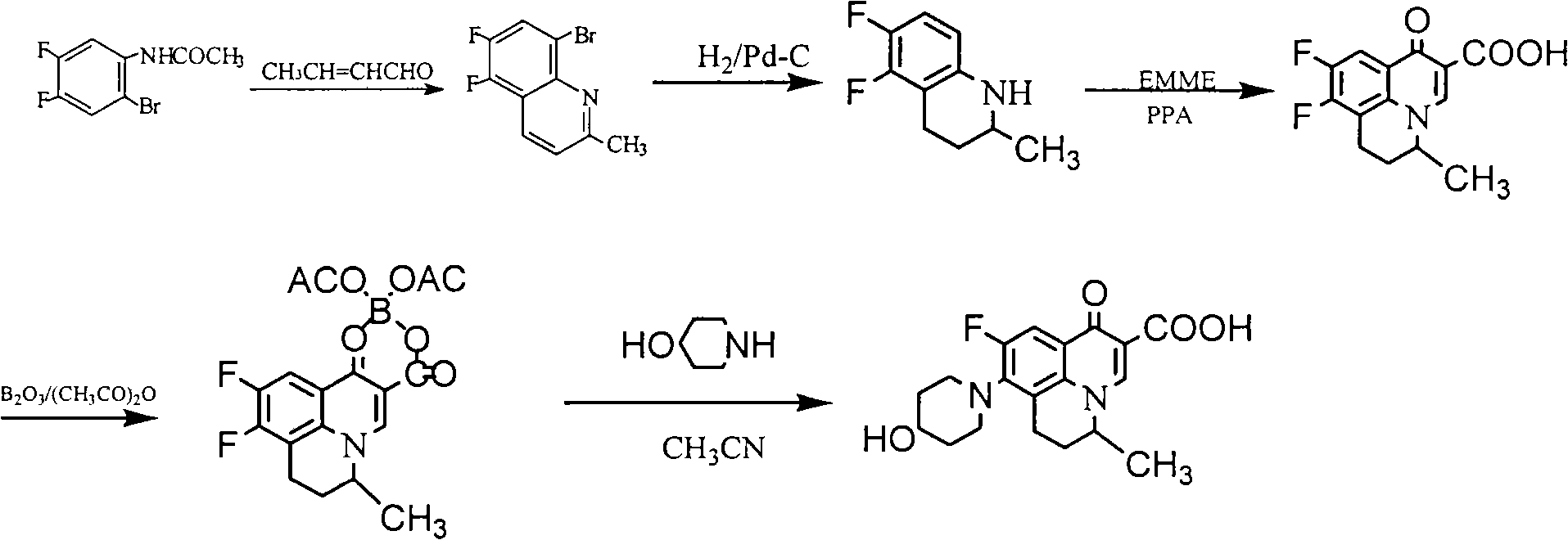
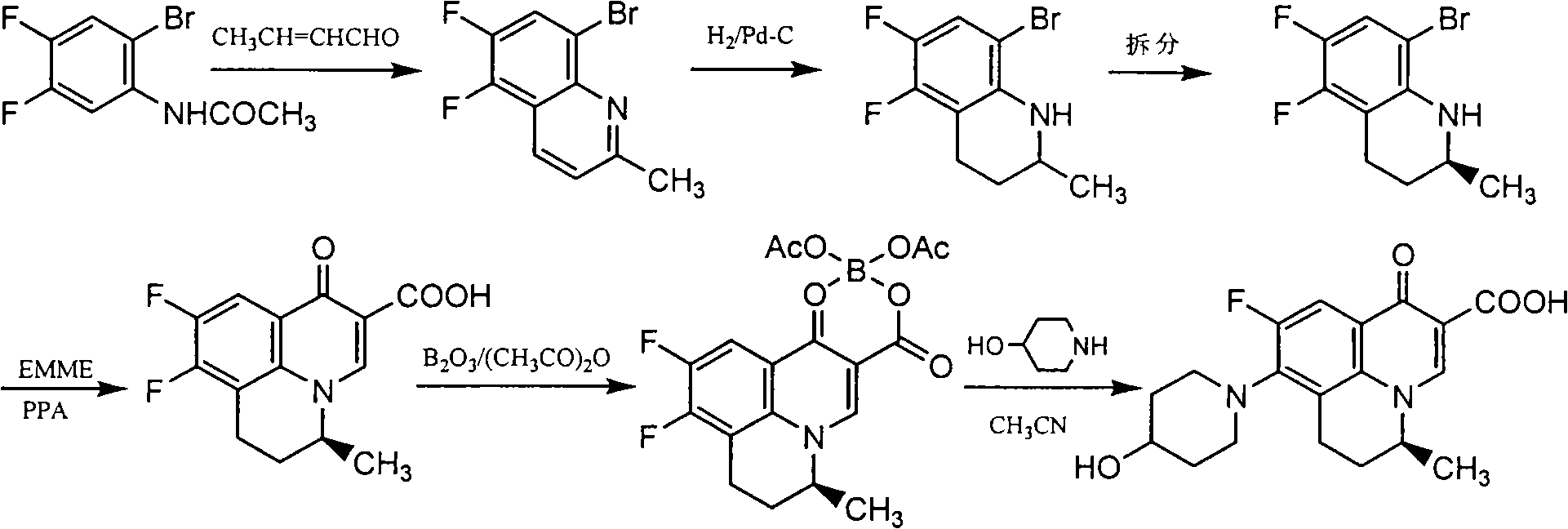
[0040] Example 2: Preparation of S-(-)-5,6-difluoro-2-methyl-1,2,3,4-tetrahydroquinoline (Method 1)
[0041]
[0042] Add 98 g (0.27 mol) of L-2,3-dibenzoyl tartaric acid to 200 mL of ethyl acetate and stir at room temperature to dissolve, then add (±)-5,6-difluoro-2-methyl-1,2 dropwise while stirring, 100 g (0.55 mol) of 3,4-tetrahydroquinoline, after dropping, stir at room temperature for 1 hr, collect the precipitated solid by filtration, wash with ethyl acetate, recrystallize with 70% ethanol, add 500 mL of water and 500 mL of ethyl acetate to the obtained solid and mix In the solution, adjust the pH to 9-10 with 20% NaOH solution under stirring, separate the organic layer, wash with saturated NaCl solution, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain 38 g of light yellow oil. Yield: 38%, m.p.: 29-30°C, [a] D 20 =-36.4 (C=0.5, methanol), M+1: 184.09. Elemental analysis, calculated...
Embodiment 3
[0043] Example 3: Preparation of S-(-)-5,6-difluoro-2-methyl-1,2,3,4-tetrahydroquinoline (method 2)
[0044] Add 98 g (0.27 mol) of L-2,3-dibenzoyl tartaric acid to 200 mL of acetone and stir at room temperature to dissolve, then add (±)-5,6-difluoro-2-methyl-1,2,3 dropwise under stirring, 100 g (0.55 mol) of 4-tetrahydroquinoline, after dropping, stir at room temperature for 1 hr, collect the precipitated solid by filtration, wash with ethyl acetate, recrystallize with 70% ethanol, add the obtained solid to a mixed solution of 500 mL of water and 500 mL of ethyl acetate , adjust pH=9-10 with 20% NaOH solution under stirring, separate the organic layer, wash with saturated NaCl solution, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain 37.5 g of light yellow oil. Yield: 37.5%, m.p.: 29-30°C, [a] D 20 =-36.6 (C=0.5, methanol), M+1: 184.09. Elemental analysis, calculated value (%): C, 65.56; H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com