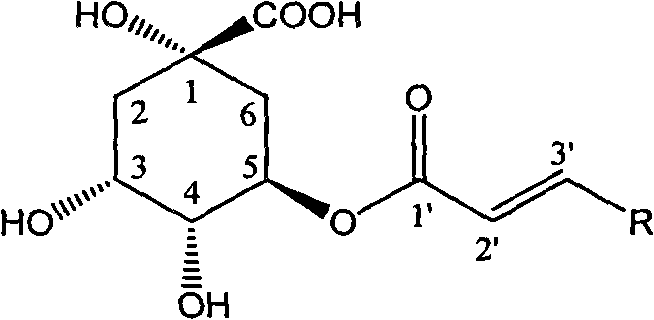
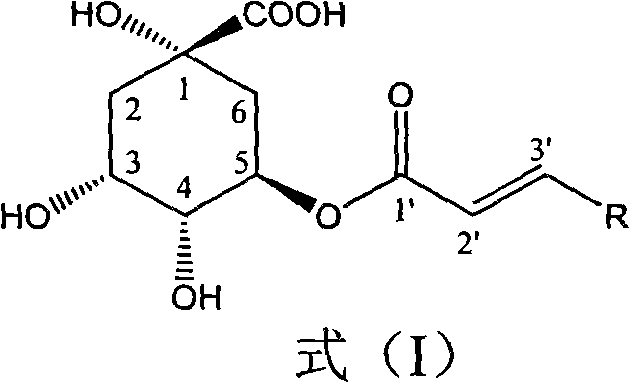
5-oxygen-substituted benzene alkene propionyl quinic acid compounds and medicine uses thereof
A technology of phenylacryloyl and quinic acid, which is applied in the field of 5-oxy-[3-substituted phenylacryloyl] quinic acid compounds and their preparation, and can solve problems such as side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 : Compound I-a is the preparation of 5-oxo-[3-(4-methoxyphenyl) acryloyl]-quinic acid
[0022] Add p-methoxyphenylpropenic acid (103 mg, 0.58 mmol), carbonyldiimidazole (119 mg, 0.58 mmol) and 8 ml of tetrahydrofuran in the reaction flask, reflux for 2 hours, then add quinic acid (90 mg, 0.47 mmol), 1,8-diazabicyclo[5,4,0]11-alk-7-ene (DBU, 90 mg, 0.58 mmol), and the whole solution was refluxed for another 8 hours. Precipitation gave a pale yellow viscous solid, which was separated by reverse phase silica gel RP-18 column chromatography (methanol / water: 50 / 50) to obtain 43 mg of white solid with a yield of 26.7%.
[0023] Melting point: 122~124℃, R f (chloroform / methanol / formic acid: 50 / 2 / 1): 0.35; H NMR spectrum ( 1 HNMR, 400MHz, deuterated methanol): δ2.16~2.36 (4H, multiplet), 3.83 (3H, singlet), 4.26 (1H, double doublet), 4.55 (1H, multiplet), 4.98 (1H, doublet), 6.35 (1H, doublet), 6.82 (2H, singlet), 7.27 (2H, singlet), 7.69 (1H, doublet).
Embodiment 2
[0024] Example 2 : Compound I-a is the preparation of 5-oxo-[3-(4-methoxyphenyl) acryloyl]-quinic acid
[0025]
[0026] Add p-methoxyphenylacrylate (87 mg, 0.49 mmol), dicyclohexylcarbodiimide (100 mg, 0.49 mmol) and 8 ml of dichloromethane into the reaction flask, and stir at room temperature for 20 minutes , add quinic acid (75 mg, 0.39 mmol), 4-dimethylaminopyridine (DMAP, 10 mg, 0.049 mmol), the whole solution was reacted overnight at room temperature, and the insoluble matter was removed by suction filtration, the solvent was evaporated, and the Dissolved to obtain a white solid, which was separated by reverse-phase silica gel RP-18 column chromatography (methanol / water: 50 / 50) to obtain 38 mg of white solid with a yield of 28.6%.
Embodiment 3-8
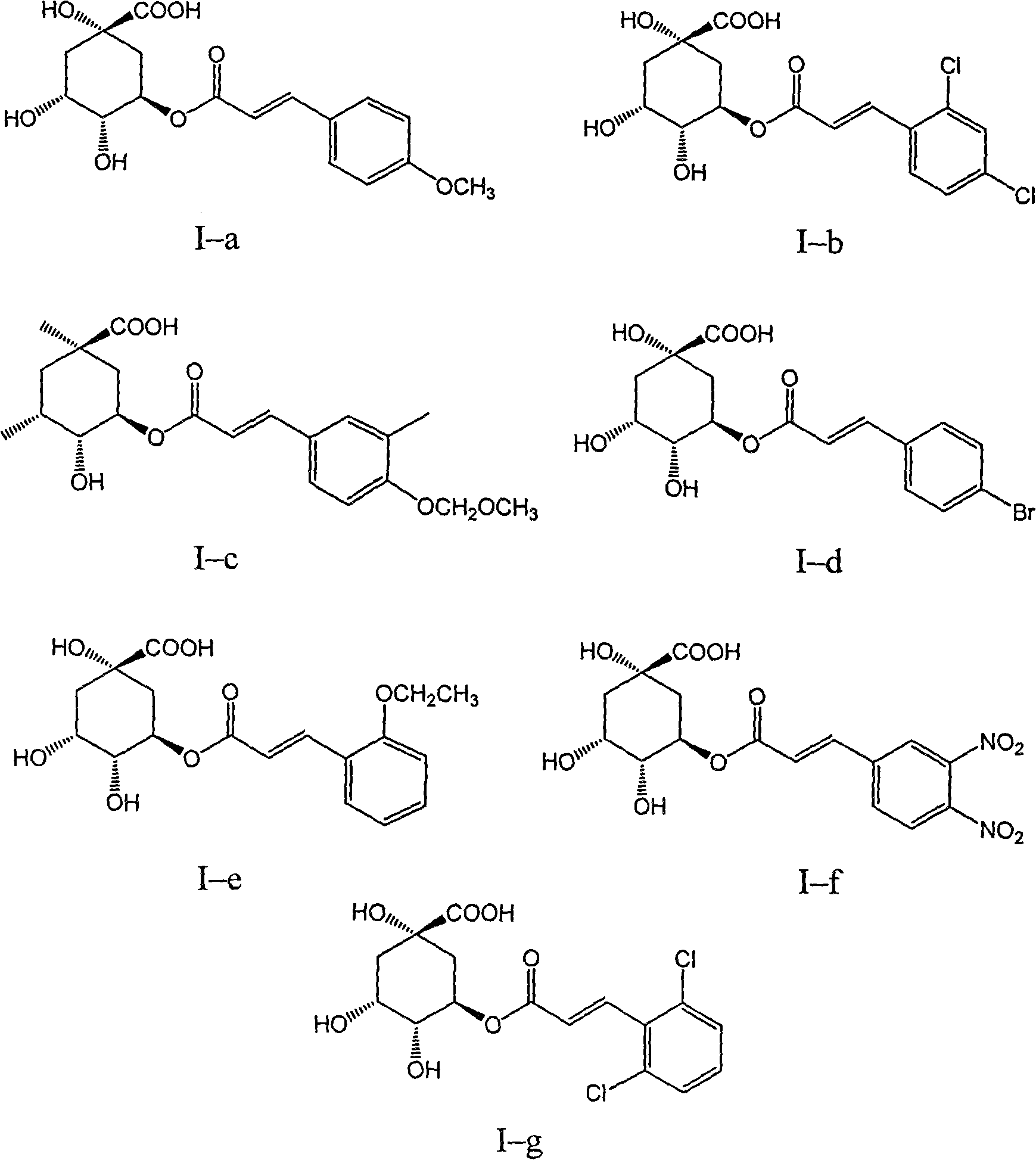
[0027] According to the same method as in Example 2, the compounds of Example 3-8 shown in Table 1 were prepared:
[0028]
[0029] Table I
[0030]
[0031]
[0032] List the physicochemical data of each compound in Table 1 below:
[0033] Compound I-b: white solid, melting point: 132-135°C, R f (chloroform / methanol / formic acid: 50 / 2 / 1): 0.23; H NMR spectrum ( 1 HNMR) (400MHz, deuterated methanol): δ2.09~2.28 (4H, multiplet), 3.93 (1H, double doublet), 4.09 (1H, multiplet), 4.93 (1H, double doublet), 6.28( 1H, bimodal), 7.32 (1H, multiplet), 7.49 (1H, bimodal), 7.56 (1H, unimodal), 7.92 (1H, bimodal).
[0034] Compound I-c: white solid, melting point: 110-113°C, R f (chloroform / methanol / formic acid: 50 / 2 / 1): 0.38; H NMR spectrum ( 1HNMR) (400MHz, deuterated methanol): δ2.16~2.36 (4H, multiplet), 3.48 (3H, singlet), 3.51 (3H, singlet), 3.78 (1H, double doublet), 4.10 (1H , multiplet), 4.93 (1H, double doublet), 5.18 (2H, singlet), 5.26 (2H, singlet), 6.37 (1H, d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com