Low-temperature catalytic benzene hydrogenation method and special catalyst thereof
A catalyst, a technology for catalyzing benzene, applied in the direction of physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve problems that do not involve cobalt catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Preparation of alumina-supported platinum-cobalt bimetallic catalyst and its catalytic performance for benzene hydrogenation
[0026] 1. Preparation of alumina-supported platinum-cobalt bimetallic catalyst
[0027] 0.12g Pt(NH 3 ) 4 (NO 3 ) 2 and 7.4g Co(NO 3 ) 2 ·6H 2 O was dissolved in 200 mL of deionized water, and 3.5 g of Al was added 2 o 3 The carrier, after magnetically stirring for 2 hours, was evaporated to dryness in a water bath at 95°C, then baked in an oven at 110°C for 12 hours, ground and calcined in a muffle furnace at 500°C for 4 hours to obtain a platinum-cobalt bimetallic catalyst supported on alumina.
[0028] 2. Benzene hydrogenation activity test and results
[0029] 1. Activation of the catalyst
[0030] Put 100 mg of alumina-supported platinum-cobalt bimetallic catalyst into a U-shaped quartz tube, and perform a reduction reaction with hydrogen-nitrogen mixed gas at 450 °C for 1 h, and the flow rates of hydrogen and nitrogen ...
Embodiment 2
[0034] Example 2. Preparation of silica-supported platinum-cobalt bimetallic catalyst and its catalytic performance for benzene hydrogenation
[0035] 1. Preparation of silica-supported platinum-cobalt bimetallic catalyst
[0036] 0.12g Pt(NH 3 ) 4 (NO 3 ) 2 and 7.4gCo(NO 3 ) 2 ·6H 2 O was dissolved in 200 mL of deionized water, and 3.5 g of SiO 2 The carrier, after being magnetically stirred for 2 hours, was evaporated to dryness in a water bath at 95°C, then baked in an oven at 110°C for 12 hours, ground and calcined in a muffle furnace at 500°C for 4 hours to obtain a silica-supported platinum-cobalt bimetallic catalyst.
[0037] 2. Benzene hydrogenation activity test and results
[0038] 1. Activation of the catalyst
[0039] Put 100 mg of silica-supported platinum-cobalt bimetallic catalyst into a U-shaped quartz tube, and perform reduction reaction with hydrogen-nitrogen mixed gas at 450° C. for 1 h, and the flow rates of hydrogen and nitrogen are both 20 mL / min. ...
Embodiment 3
[0045] Example 3. Preparation of alumina-supported cobalt metal catalyst and its catalytic performance for benzene hydrogenation
[0046] 1. Preparation of alumina-supported cobalt metal catalyst
[0047] 7.4g Co(NO 3 ) 2 ·6H 2 O was dissolved in 200 mL of deionized water, and 3.5 g of Al was added 2 o 3 The carrier, after magnetically stirring for 2 hours, was evaporated to dryness in a water bath at 95°C, then baked in an oven at 110°C for 12 hours, ground and calcined at 500°C for 4 hours in a muffle furnace to obtain an alumina-supported cobalt metal catalyst.
[0048] 2. Benzene hydrogenation activity test and results
[0049] Same as Step 2 of Example 1.
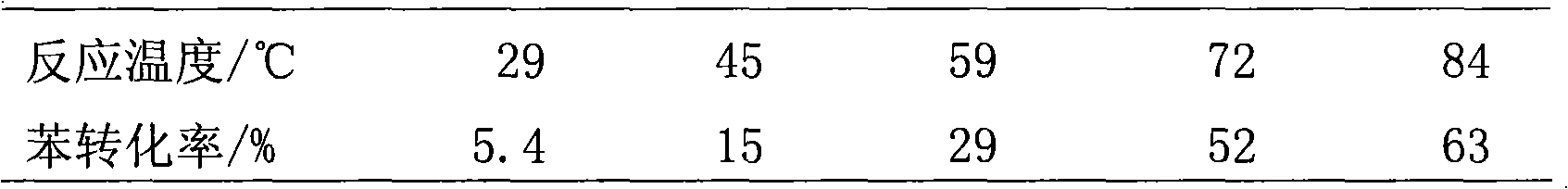
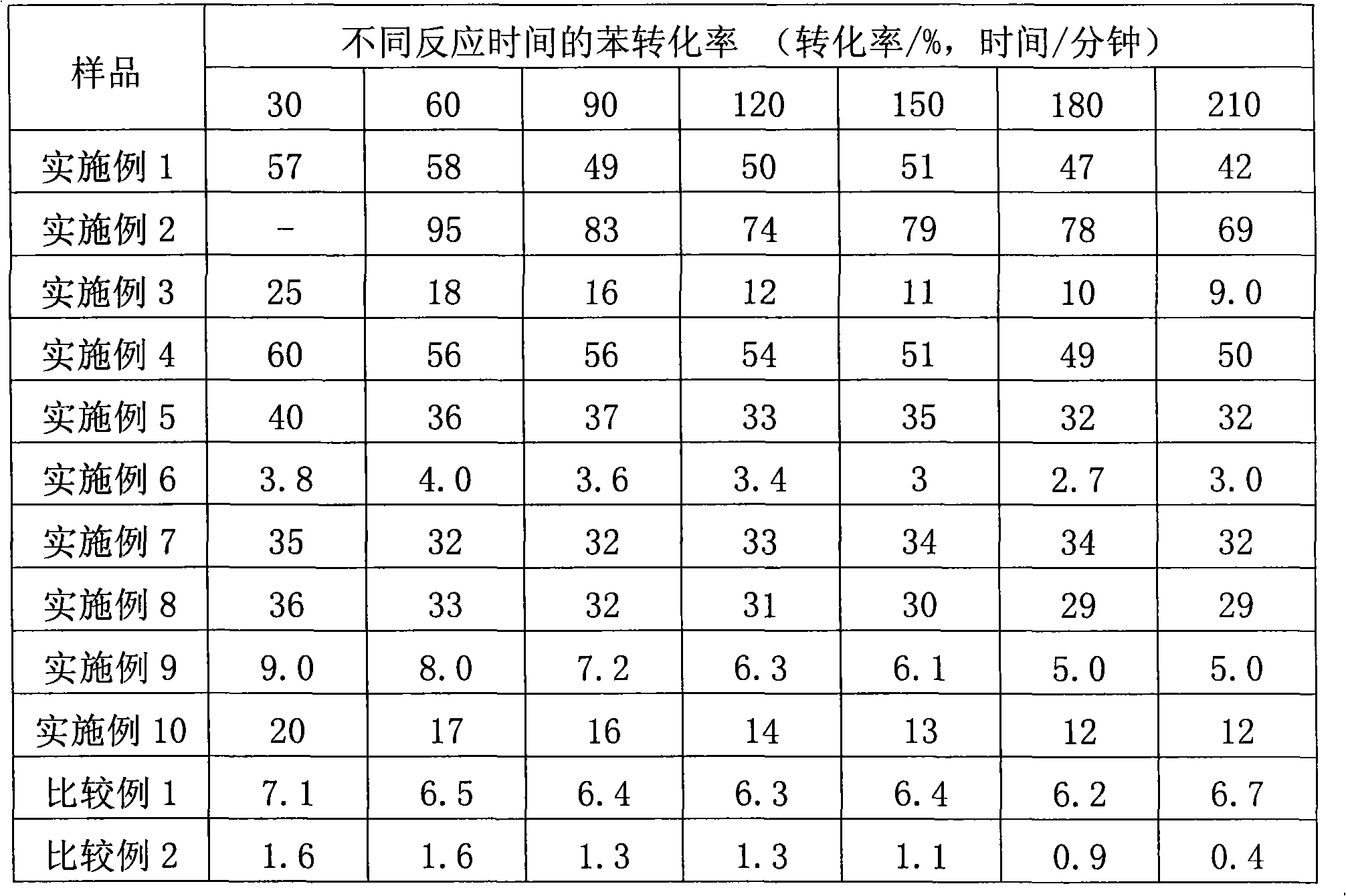
[0050] The results of the benzene hydrogenation reaction are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com