Process and device for preparing H2 and CO by co-transformation of CH4 and CO2
A technology of co-conversion of CO2, applied in chemical instruments and methods, inorganic chemistry, carbon monoxide, etc., can solve the problems of low synthesis gas yield, carbon deposition and deactivation, high requirements, etc., to overcome low conversion rate, high efficiency conversion, The effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
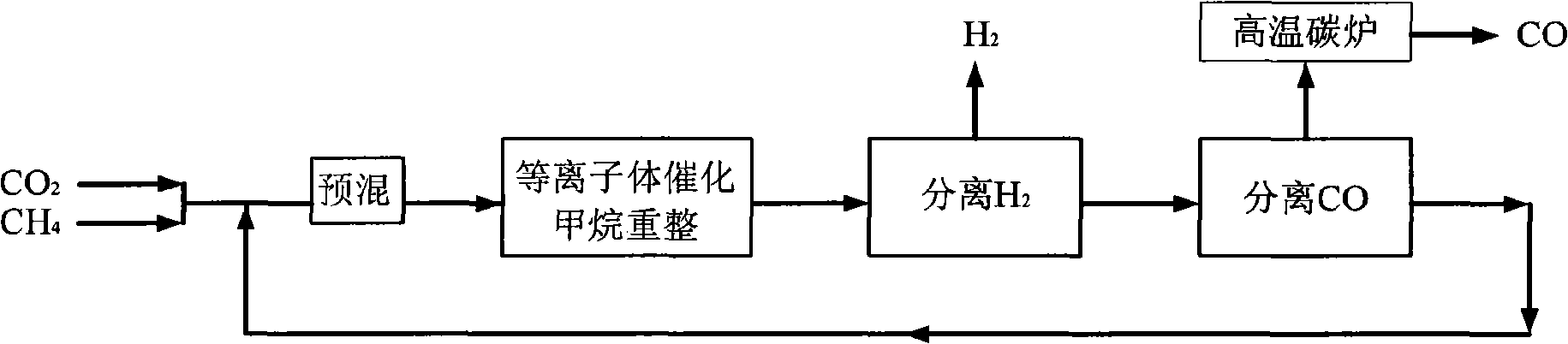
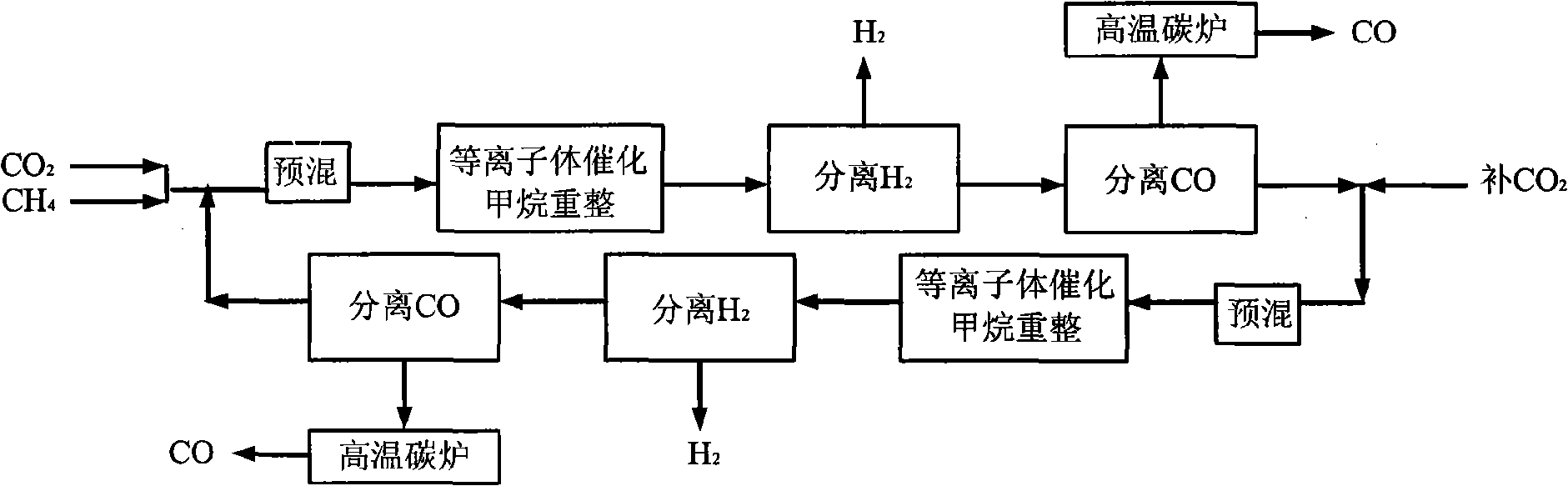
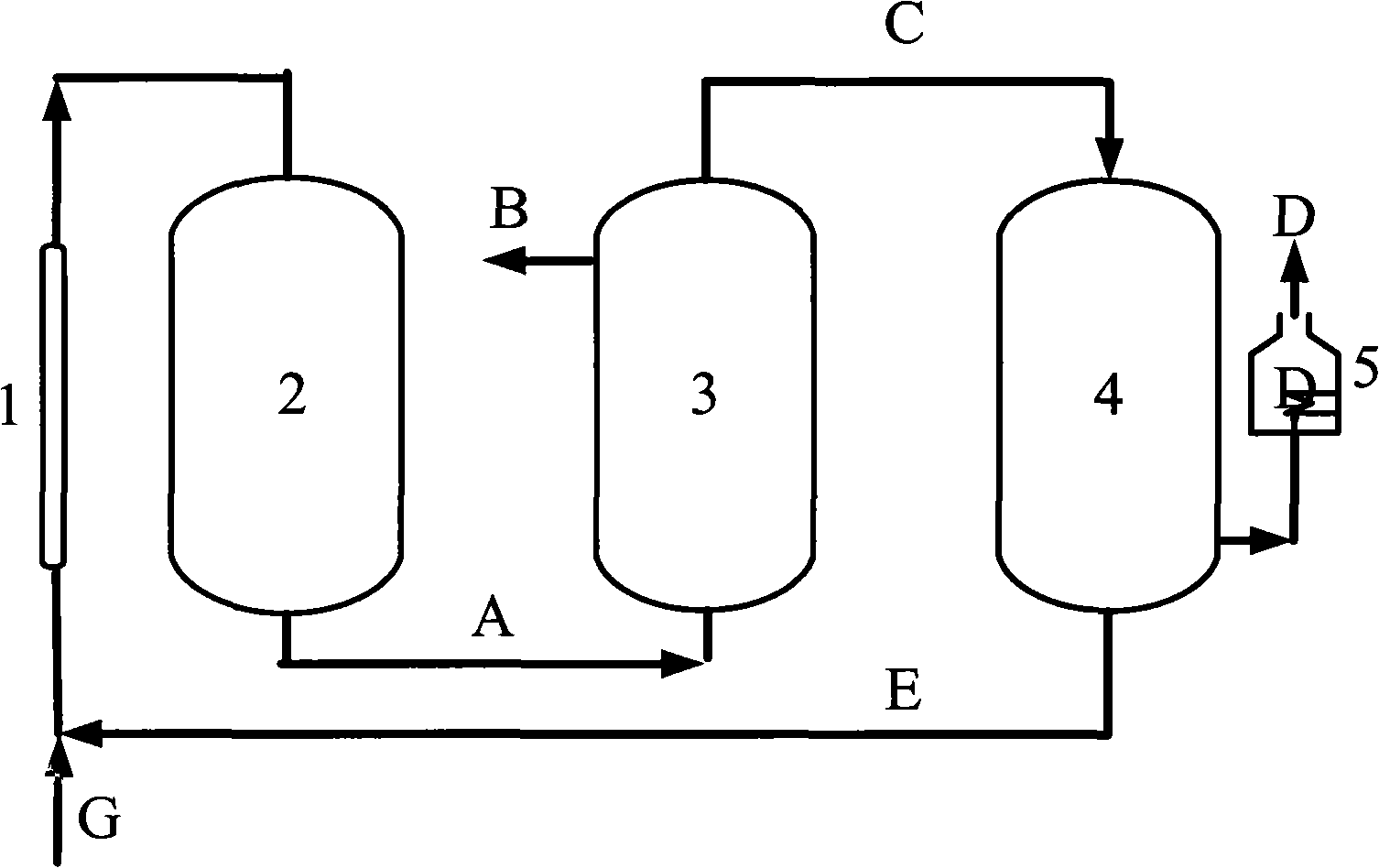
[0036] CH 4 with CO 2 Feed at a molar ratio of 1:1, the reactor operating pressure is 7 atm, in the low temperature plasma reactor, CH 4 The conversion rate reaches 50%, CO 2 The conversion rate reaches 27%, the selectivity of CO is about 60%, and the H 2 The selectivity is about 80%, the remaining gas is C 2 h 6 , C 2 h 4 , C 3 h 8 High carbon hydrocarbons, this mixed gas enters the H 2 Separator, most H 2 is separated, the purity reaches 90%-95%, and the remaining gas enters the CO separator, almost all of the CO and a part of the CO 2 are separated, and a small amount of CH 4 and the remaining H 2 They are also separated, and they enter the high-temperature carbon furnace for burning together to obtain CO gas with a purity of more than 95%. Most of the remaining gas after passing through the CO separator is CH 4 , a small part is CO 2 , they return to the low-temperature plasma reactor together with the raw material gas for the next reaction-separation-separat...
Embodiment 2
[0038] CH4 and CO2 are fed in a molar ratio of 4:1, and the operating pressure of the reactor is 7atm. In the low temperature plasma reactor, CH 4 The conversion rate reaches 14%, CO 2 The conversion rate reaches 42%, the selectivity of CO is about 80%, and the H 2 The selectivity is about 60%, the remaining gas is C 2 h 6 , C 2 h 4 , C 3 h 8 High carbon hydrocarbons, this mixed gas enters the H 2 Separator, most H 2 is separated, the purity reaches 90%-95%, and the remaining gas enters the CO separator, all CO and all CO 2 are separated, and a small amount of CH 4 and the remaining H 2 They are also separated, and they enter the high-temperature carbon furnace for burning together to obtain CO gas with a purity of more than 95%. Almost all of the remaining gas after passing through the CO separator is CH 4 , they return to the low-temperature plasma reactor together with the raw material gas for the next reaction-separation-separation process.
Embodiment 3
[0040] CH4 and CO2 are fed in a molar ratio of 2:1, and the operating pressure of the reactor is 7atm. In the low-temperature plasma reactor, CH 4 The conversion rate reaches 22%, CO 2 The conversion rate reaches 32%, the selectivity of CO is about 64%, H 2 The selectivity is about 70%, the remaining gas is C 2 h 6 , C 2 h 4 , C 3 h 8 High carbon hydrocarbons, this mixed gas enters the H 2 Separator, most H 2 is separated, the purity reaches 90%-95%, and the remaining gas enters the CO separator, all CO and all CO 2 are separated, and a small amount of CH 4 and the remaining H 2 They are also separated, and they enter the high-temperature carbon furnace for burning together to obtain CO gas with a purity of more than 95%. Almost all of the remaining gas after passing through the CO separator is CH 4 , they return to the low-temperature plasma reactor together with the raw material gas for the next reaction-separation-separation process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com