Method for atmospheric digestion of laterite ore
A technology of ore and laterite, applied in the field of hydrometallurgy, can solve the problem of increasing acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A mixture of limonite and saprolite ores having a dry ratio of about 1 is formed into an aqueous slurry. The aqueous slurry is then mixed with 93% concentrated sulfuric acid to form a leach slurry. The acid was dosed in excess of 100% of the stoichiometric amount required to dissolve 90% of the nickel and cobalt in the mixed ore fraction. The first leaching process is carried out in a single reactor at a temperature of at least 80°C for at least 2 hours. During the first leaching process, iron compounds are precipitated out of solution.
[0044] The overflow from the leaching process is sent to a second reactor where the saprolite ore slurry is added to the mixture. A second leaching process is then performed, also at a temperature of at least 80°C for at least about 2 hours. During the second leaching process, iron compounds are further precipitated out of solution.
[0045] After the second leaching process is complete, the solid residue is separated from the leac...
Embodiment 3
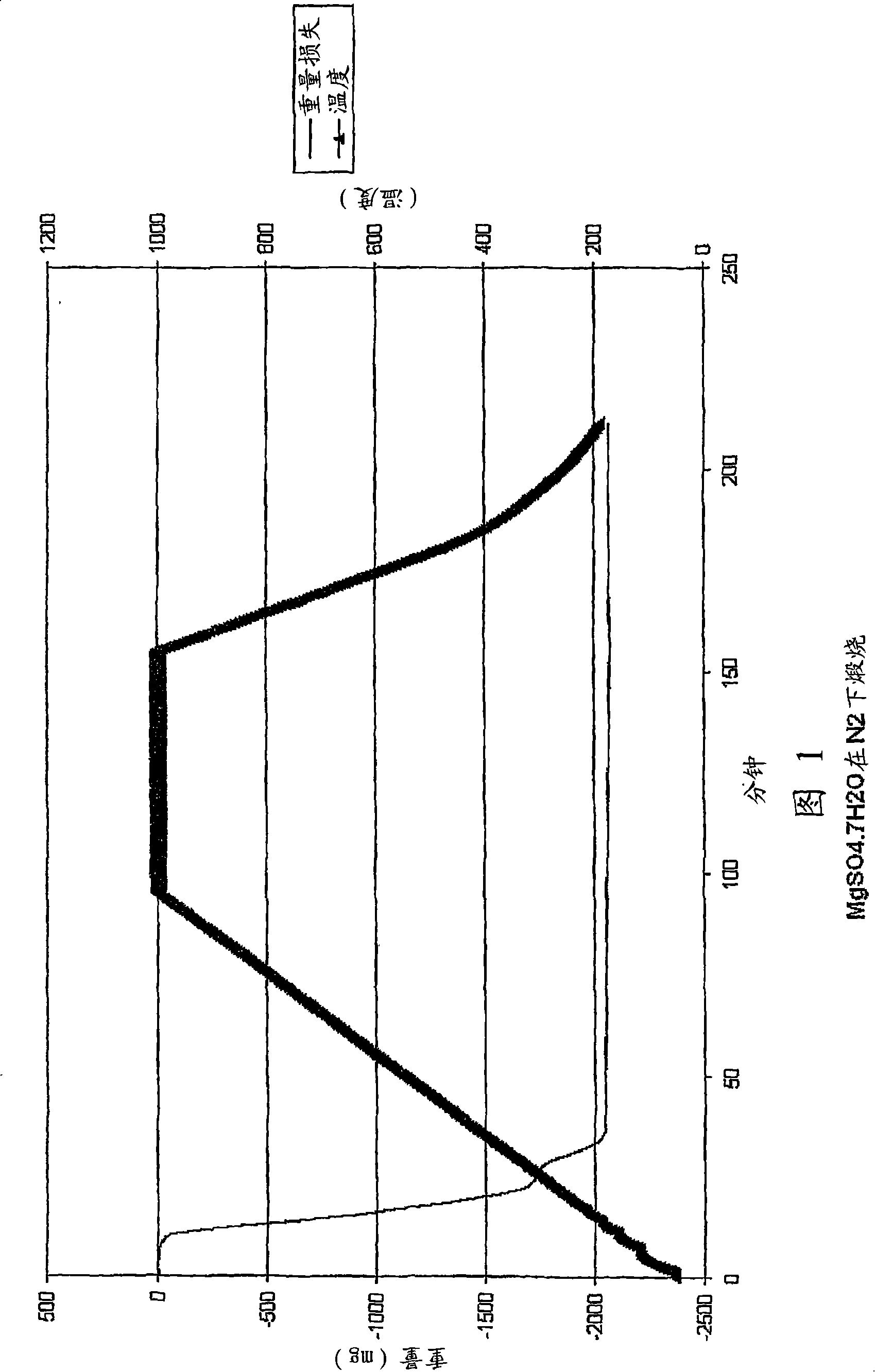
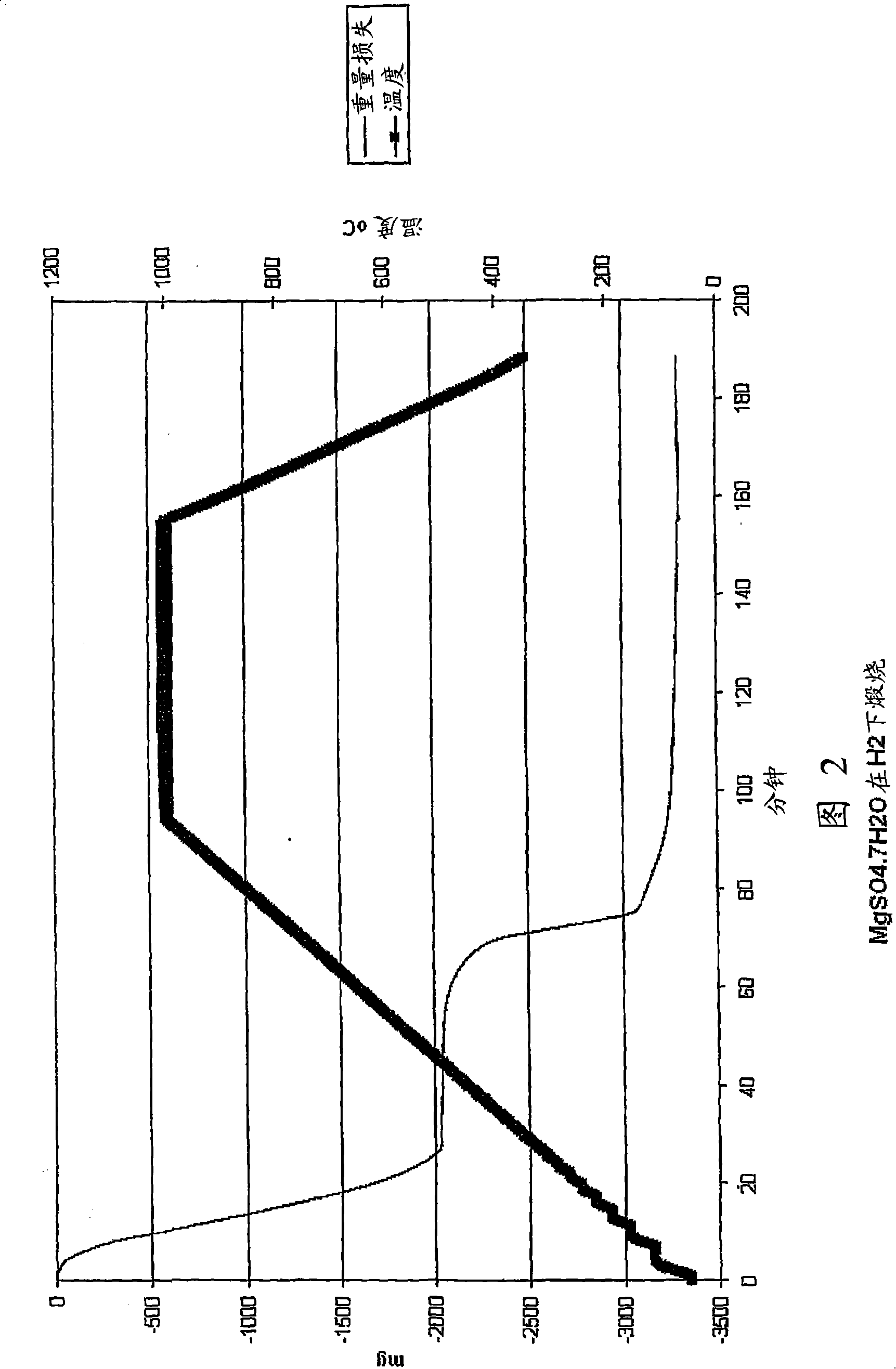
[0053] A sample of magnesium sulfate heptahydrate (4.0093 g) was placed in a small basket and calcined in a thermogravimetric analyzer (TGA) under dry hydrogen flow (5 L / min). The temperature in TGA also increased from room temperature to 1000°C at a rate of 10°C / min. The sample showed a weight loss of about 2.03 g to 350°C, corresponding to the loss of water of crystallization. The weight then remained stable until 630°C, at which time a further weight loss was about 1.06 g. The rapid weight loss slows down at a temperature of 810°C. By 1000°C, the total weight loss had reached about 3.29 g. The remaining sample was carefully removed from the container and weighed. The weighed sample mass (0.63g) is very close to the formula MgO (theoretical weight 0.655g). A diagram of this process is shown in Figure 2.
Embodiment 4
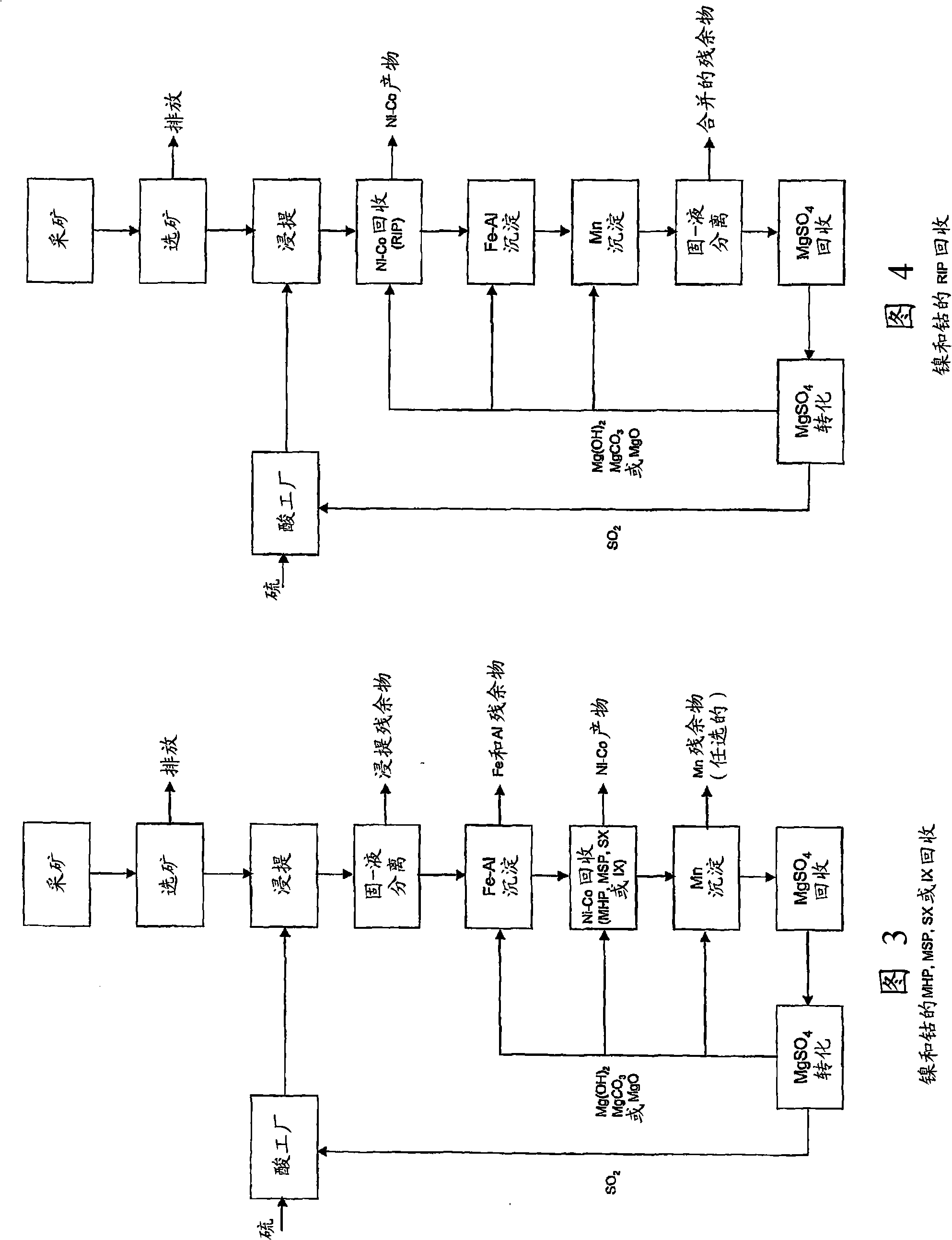
[0055] Example 4 is a flow diagram shown in Figure 3 showing the process stages in an embodiment of the invention. In this example, separation of the leach residue from the enriched leach solution occurs prior to removal of residual iron and aluminum and recovery of Ni and Co metals. Ni and Co recovery is achieved using a technique selected from mixed hydroxide precipitation, mixed sulfide precipitation, solvent extraction or ion exchange.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com