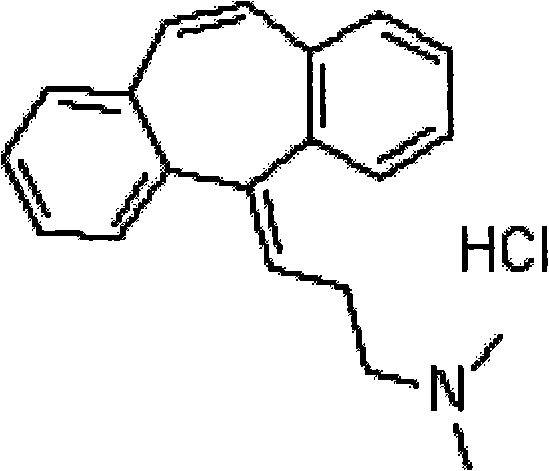
Method for refining cyclobenzaprine hydrochloride
A technology of cyclobenzaprine hydrochloride and a purification method is applied in the field of purification for obtaining off-white cyclobenzaprine hydrochloride, can solve problems such as limited application of acetic anhydride, unfavorable industrialization, etc., and achieves the effects of improving yield and increasing safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
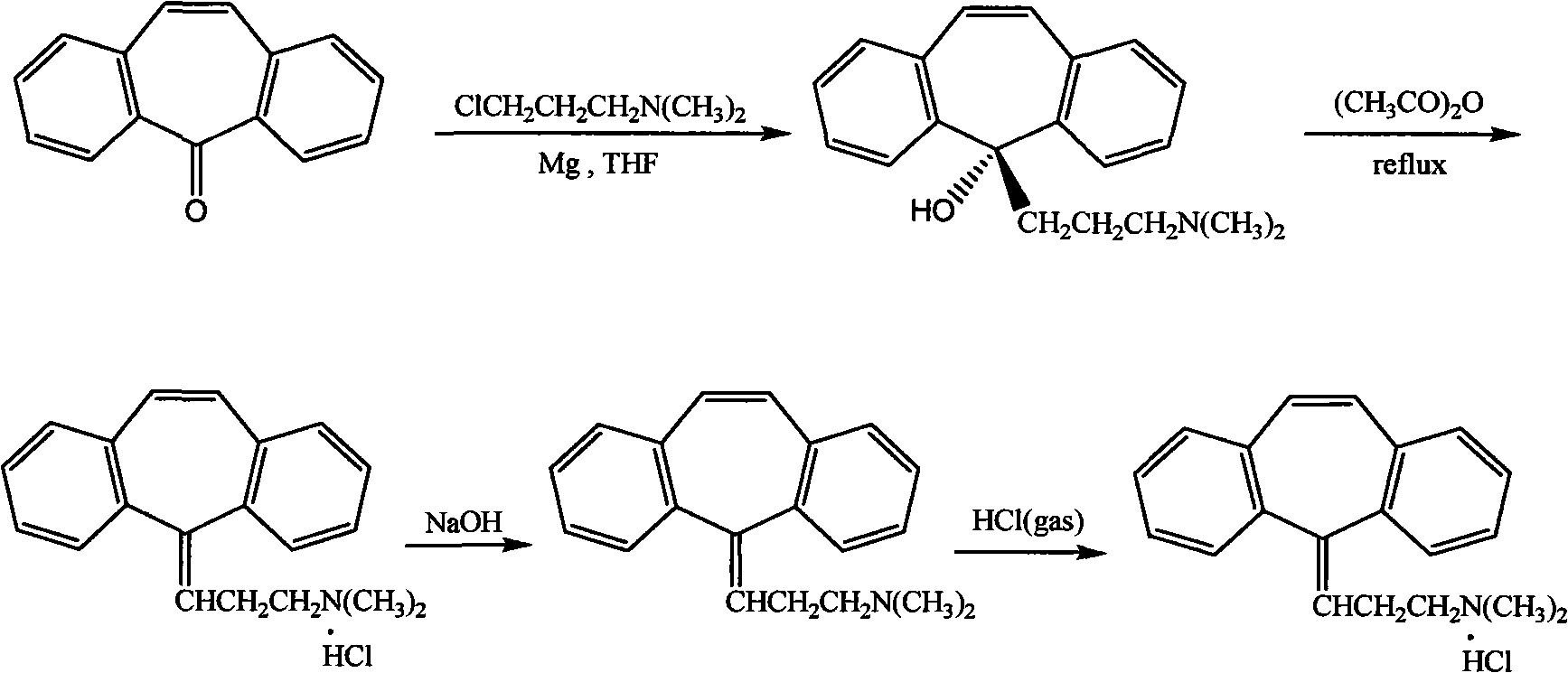
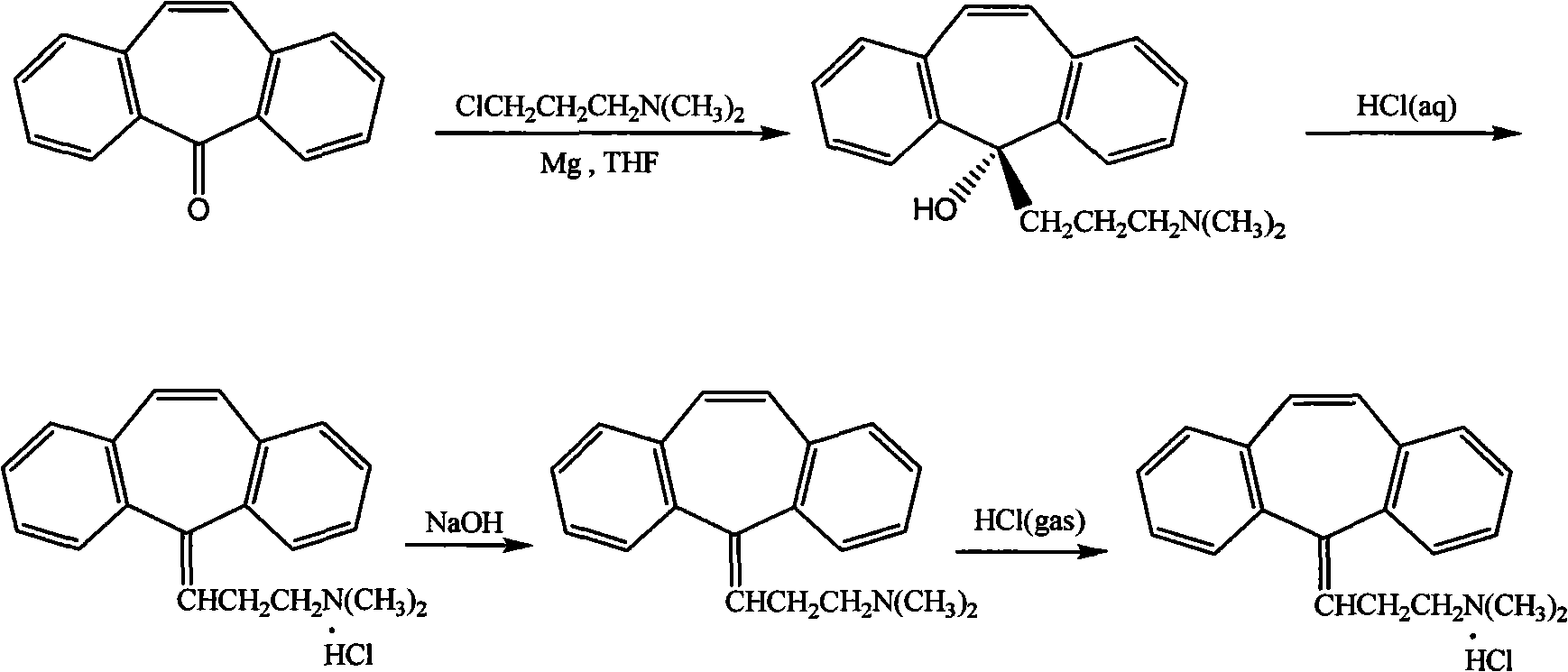
[0026] In the first step, the crude product of 5-(3-dimethylaminopropyl)-dibenzo[a,d]cycloheptatrien-5-ol (cyclobenzaprene intermediate) was dissolved in 4% hydrochloric acid, Heat at 90°C for 2 hours, add excess sodium hydroxide to neutralize and make the solution alkaline; in the second step, extract with ether / ethyl acetate mixture (volume ratio 8:2), and pass dry hydrogen chloride gas into the organic layer for 1 Hour, cyclobenzaprine hydrochloride crude product is separated out. The third step: recrystallize the crude product of cyclobenzaprine hydrochloride with anhydrous isopropanol, filter with 30ml of anhydrous isopropanol per 10g, wash the filter cake with acetone until off-white, and dry to obtain cyclobenzaprine hydrochloride Salt boutique. The content of the obtained cyclobenzaprine hydrochloride is greater than 99.8%, and the total refining yield is 95%.
[0027] Wherein the preparation of cyclobenzaprine intermediate is as follows:
[0028] Add 20ml tetrahydr...
experiment example 2
[0030] In the first step, the crude product of 5-(3-dimethylaminopropyl)-dibenzo[a,d]cycloheptatrien-5-ol (cyclobenzaprene intermediate) (preparation is the same as in Example 1, the content (50%~80%) was dissolved in 4% phosphoric acid solution, heated at 50°C for 5 hours, and the solution was alkaline after neutralization by adding excess sodium hydroxide; 2:8) for extraction, and dry hydrogen chloride gas was passed through the organic layer for 5 hours, and the crude product of cyclobenzaprine hydrochloride was precipitated. The third step: recrystallize the crude product of cyclobenzaprine hydrochloride with anhydrous isopropanol, filter with 100ml of anhydrous isopropanol per 10g, wash the filter cake with acetone until off-white, and dry to obtain cyclobenzaprine hydrochloride Salt boutique. The content of the obtained cyclobenzaprine hydrochloride is greater than 99.7%, and the total refining yield is 95%.
Embodiment 3
[0032] In the first step, the crude product of 5-(3-dimethylaminopropyl)-dibenzo[a,d]cycloheptatrien-5-ol (cyclobenzaprene intermediate) (preparation is the same as in Example 1) is dissolved In 30% citric acid solution, heat at 65°C for 5 hours, add excess sodium carbonate to neutralize and make the solution alkaline; the second step is to extract with ether / ethyl acetate mixture (volume ratio 3:6), organic Dry hydrogen chloride gas was passed through the layer for 3 hours, and the crude product of cyclobenzaprine hydrochloride was precipitated. The third step: recrystallize the crude product of cyclobenzaprine hydrochloride with anhydrous isopropanol, filter with 50ml of anhydrous isopropanol per 10g, wash the filter cake with ether until off-white, and dry to obtain cyclobenzaprine hydrochloride Salt boutique. The content of the obtained cyclobenzaprine hydrochloride is greater than 99.9%, and the total refining yield is 96%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com