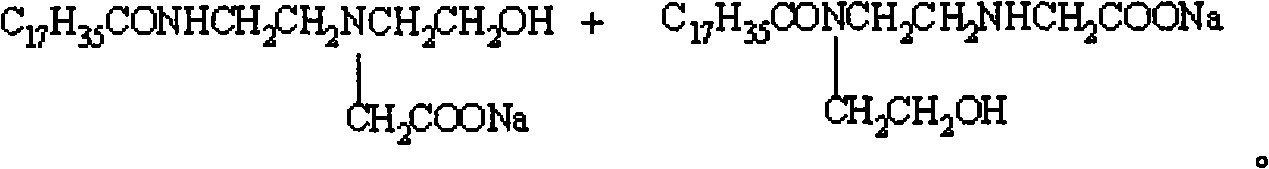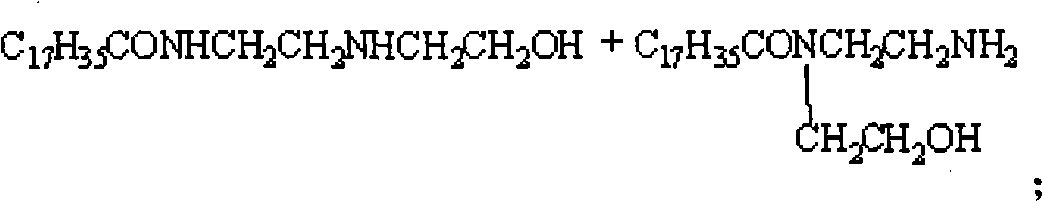Octadecanamide surfactant and synthetic method
A technology of stearic acid amide and surfactant, applied in the field of surfactant and its preparation, can solve the problems of small variety and quantity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
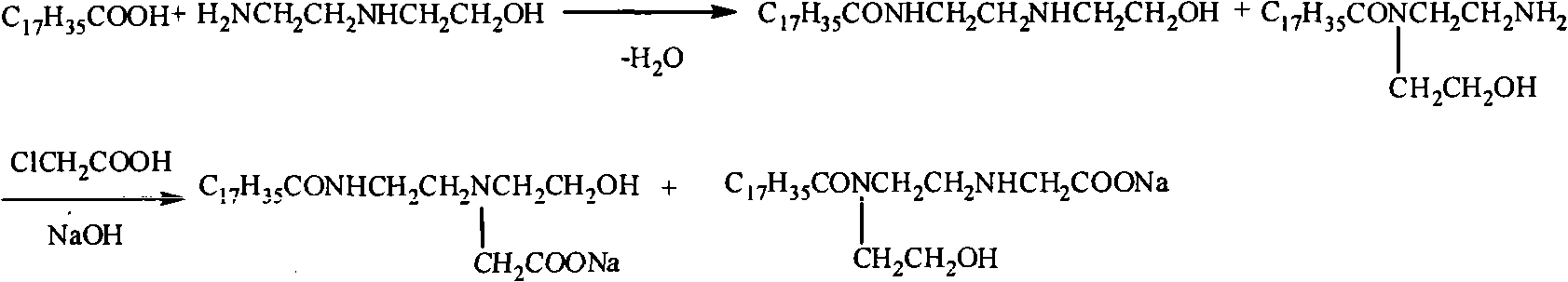
[0026] In the four-neck flask equipped with mechanical stirring, thermometer and vacuum distillation device, add stearic acid 174.2g (0.6mol) and hydroxyethylethylenediamine (molar ratio is 1: 1) and start heating and stirring, slowly The reaction temperature was raised to 100°C, and the vacuum pump was turned on to adjust the vacuum degree of the reaction system until the water came out, and the reaction temperature was gradually raised to 150°C within 3 hours until the water came out completely. Heating is stopped and the temperature is lowered to obtain the stearic acid amide surfactant intermediate.
[0027] In a four-neck flask equipped with stirring, thermometer, reflux condenser and dropping funnel, add 22.3g (0.06mol) of stearic acid amide surfactant intermediate, heat and stir, at a temperature of 50-90°C, for 4 hours Chloroacetic acid solution was added dropwise at low temperature, and then NaOH solution was added dropwise at elevated temperature. Stearic acid amide ...
Embodiment 2
[0029] In the four-neck flask equipped with mechanical stirring, thermometer and vacuum distillation device, add stearic acid 174.2g (0.6mol) and hydroxyethylethylenediamine (molar ratio is 1: 1.1) and start heating and stirring, slowly The reaction temperature was raised to 110° C., and the vacuum pump was turned on to adjust the vacuum degree of the reaction system until the water came out. The reaction temperature was gradually raised to 150° C. within 4 hours until the water came out completely. Heating is stopped and the temperature is lowered to obtain the stearic acid amide surfactant intermediate.
[0030] In a four-neck flask equipped with stirring, thermometer, reflux condenser and dropping funnel, add 22.3g (0.06mol) of stearic acid amide surfactant intermediate, heat and stir, at a temperature of 50-90°C, for 4 hours Chloroacetic acid solution was added dropwise at low temperature, and then NaOH solution was added dropwise at elevated temperature. Stearic acid amid...
Embodiment 3
[0032] In the four-neck flask equipped with mechanical stirring, thermometer and vacuum distillation device, add stearic acid 174.2g (0.6mol) and hydroxyethylethylenediamine (molar ratio is 1: 1.2) and start heating and stirring, slowly The reaction temperature was raised to 100°C, and the vacuum pump was turned on to adjust the vacuum degree of the reaction system until the water came out, and the reaction temperature was gradually raised to 150°C within 5 hours until the water came out completely. Heating is stopped and the temperature is lowered to obtain the stearic acid amide surfactant intermediate.
[0033] In a four-neck flask equipped with stirring, thermometer, reflux condenser and dropping funnel, add 22.3g (0.06mol) of stearic acid amide surfactant intermediate, heat and stir, at a temperature of 50-90°C, for 4 hours Chloroacetic acid solution was added dropwise at low temperature, and then NaOH solution was added dropwise at elevated temperature. Stearic acid amid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com