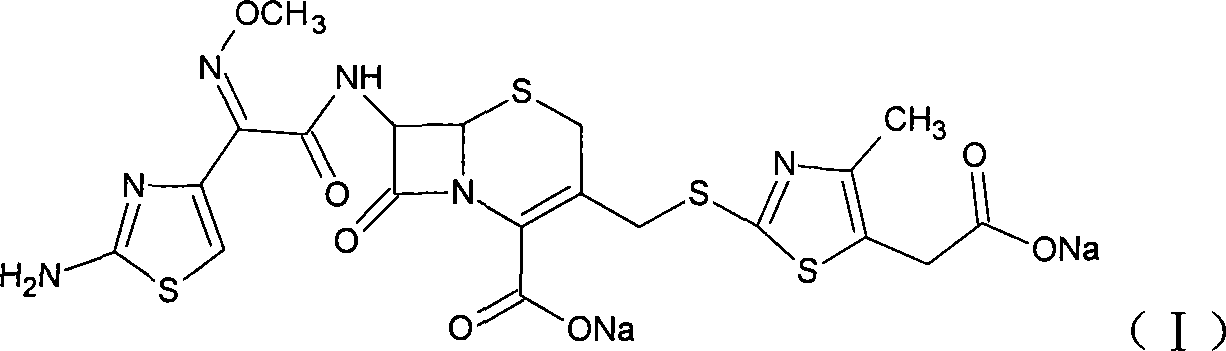
Method for preparing cefodizime sodium
A technology of cefodizime sodium and its compound, which is applied in the field of drug synthesis, can solve the problems of complex operation, low yield, and high cost, and achieve the effects of simple operation, high yield, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
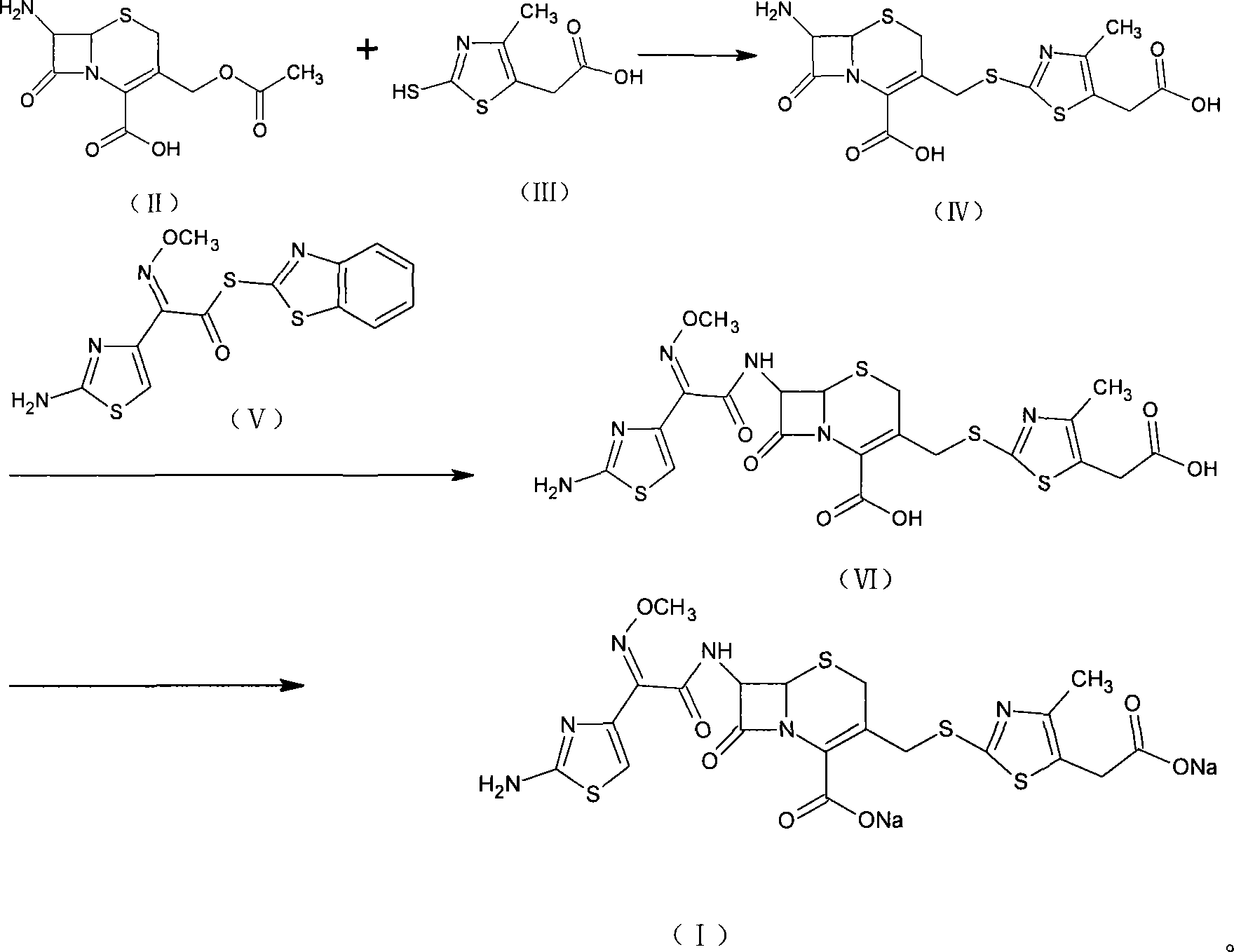
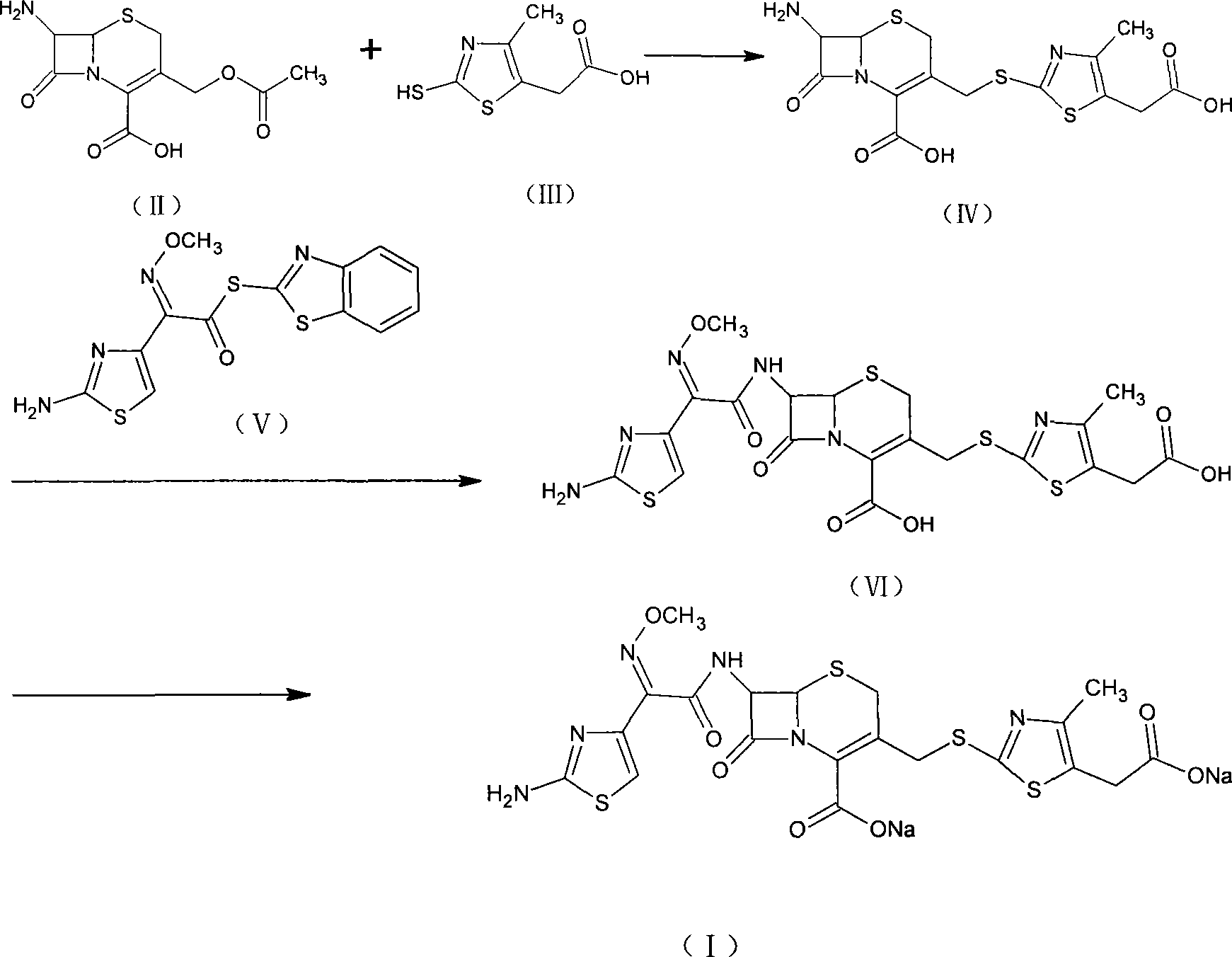
[0034] [Example 1] Synthesis of 7-amino-3-(5-carboxymethyl-4-methyl-1.3-thiazole-2-mercaptomethyl)ceph-2-ene-2-carboxylic acid (IV)
[0035] Suspend 25g of the compound of formula (II) and 20g of the compound of formula (III) in 100ml of acetonitrile, add 250ml of boron trifluoride acetonitrile, stir at room temperature for 1 hr, cool with ice water, add 300ml of water, and adjust the pH to 3.0 with ammonia. The crystals were grown for 1 hr, filtered with suction, and the filter cake was washed with 50 ml of water and 50 ml of acetone, respectively, and dried in vacuum to obtain 26.7 g (yield 72.4%, purity 98.5%) of the compound of formula (IV).
Embodiment 2
[0036] [Example 2] Synthesis of 7-amino-3-(5-carboxymethyl-4-methyl-1.3-thiazole-2-mercaptomethyl)ceph-2-ene-2-carboxylic acid (IV)
[0037] 25g of the compound of formula (II) and 20g of the compound of formula (III) were suspended in 100ml of ethyl chloroacetate, passed into 19.5g of boron trifluoride gas, stirred at room temperature for 1hr, cooled with ice water, added 200ml of water, ammoniacal liquor The pH was adjusted to 2.8, the crystals were grown for 1 hr, filtered with suction, the filter cake was washed with 50 ml of water and 50 ml of acetone, and dried in vacuum to obtain 27.5 g (73.3% yield, 98.2% purity) of the compound of formula (IV).
Embodiment 3
[0038] [Example 3] (6R, 7R)-7-[(2-amino-4-thiazolyl)-(methoxyimino)acetamido]-3-[[(5-carboxymethyl-4-methyl Synthesis of yl-2-thiazolyl)thio]methyl]-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2carboxylic acid (VI)
[0039] 26g of the compound of formula (IV) and 25g of the compound of formula (V) were suspended in 250ml of dichloromethane and 20ml of methanol, cooled with ice water, added with 18ml of triethylamine, reacted at the same temperature for 3hr, added with 200ml of water for extraction, and separated out water. Layer, add 2g activated carbon to decolorize, filter, add 3N HCl to the filtrate to adjust pH to 2.8, grow crystals for 1hr, filter with suction, and wash the filter cake with 100ml of water and 50ml of methanol respectively. Vacuum drying gave 35.5 g (yield 93.9%, purity 98.3%) of the compound of formula (VI).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com