Method for synthesizing pyrazoline derivatives capable of irradiating yellow green light
A synthesis method and pyrazoline technology are applied in the field of synthesis of pyrazoline derivatives, and can solve the problems of limiting the development and expansibility of pyrazoline compounds, low product purity, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
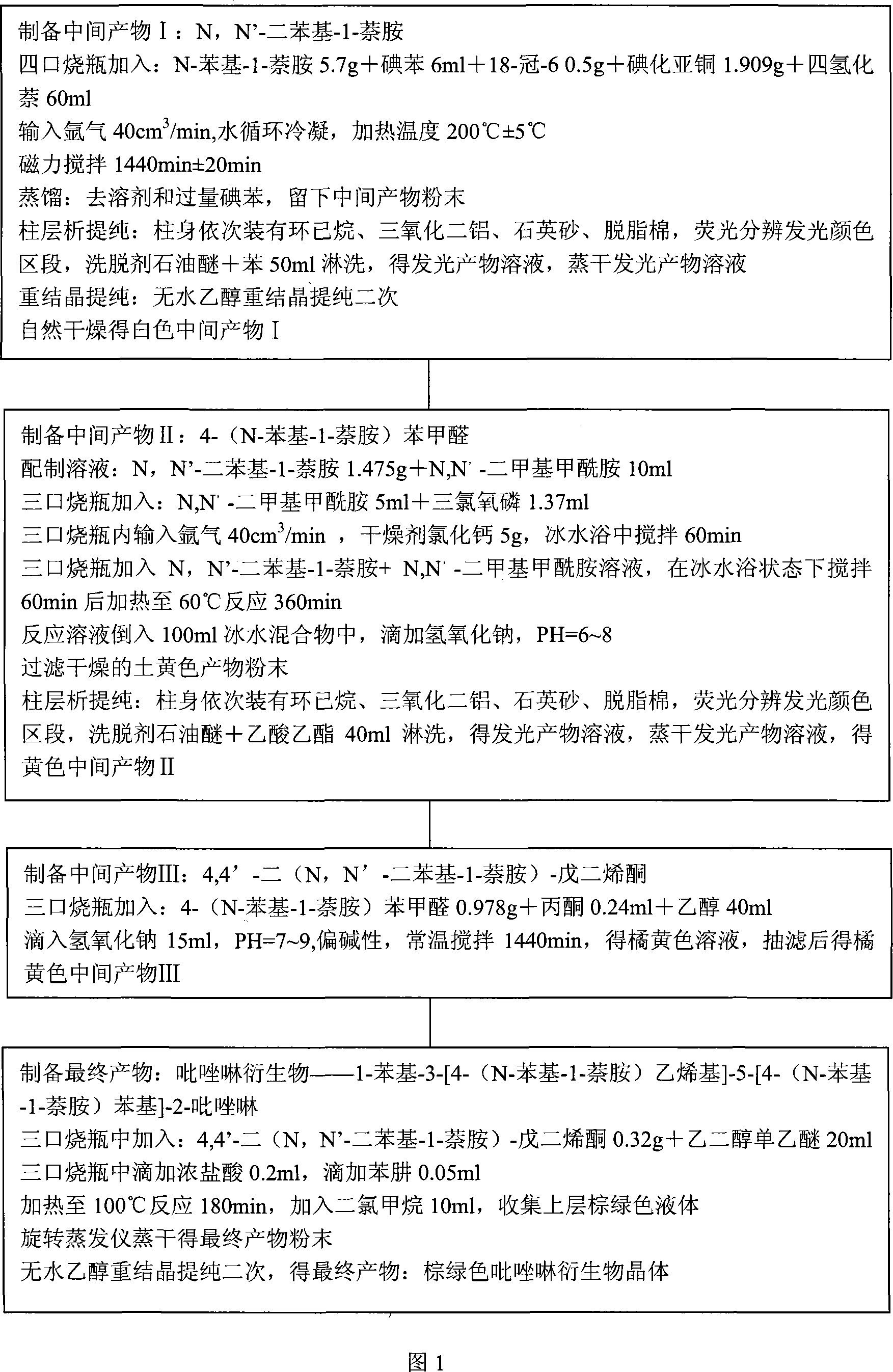
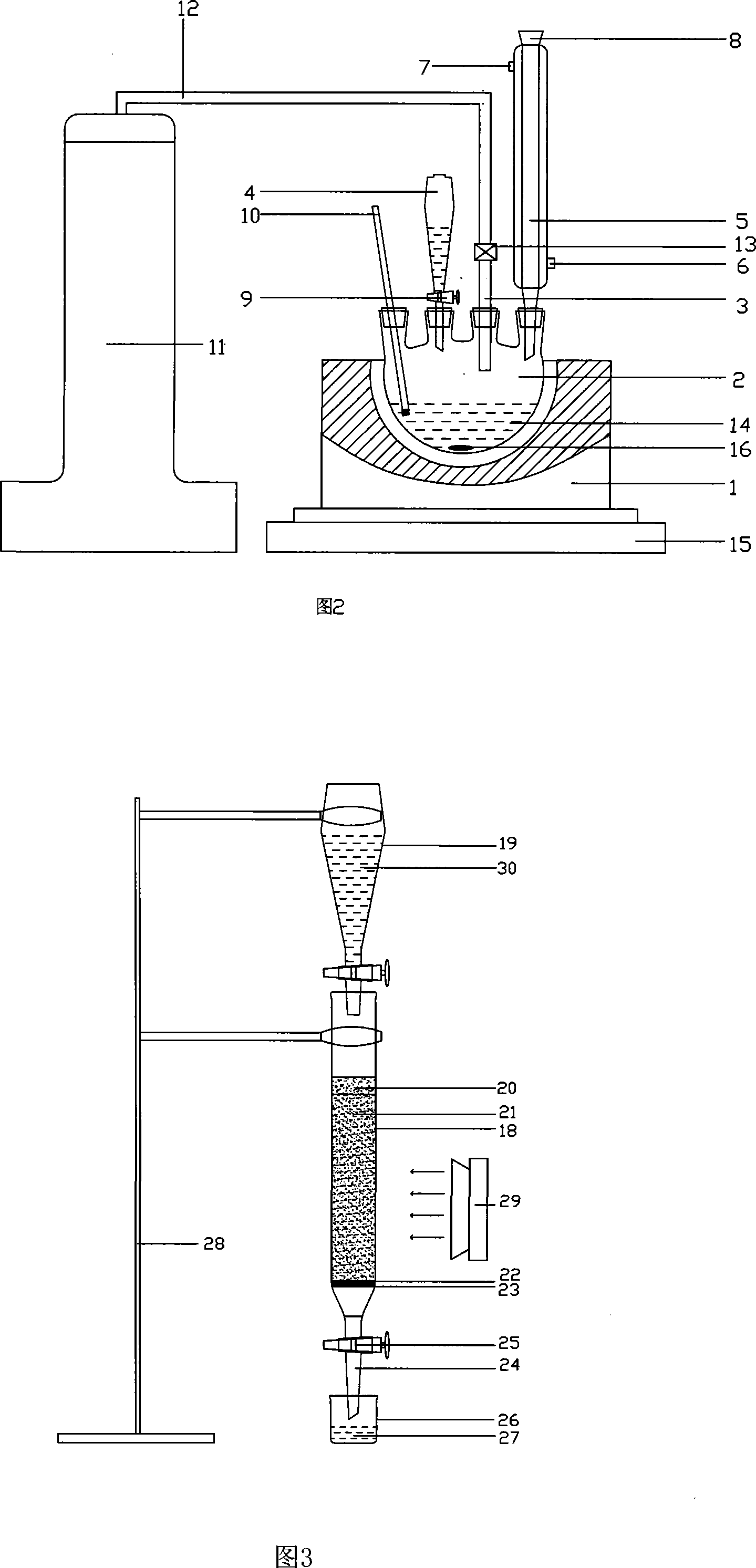
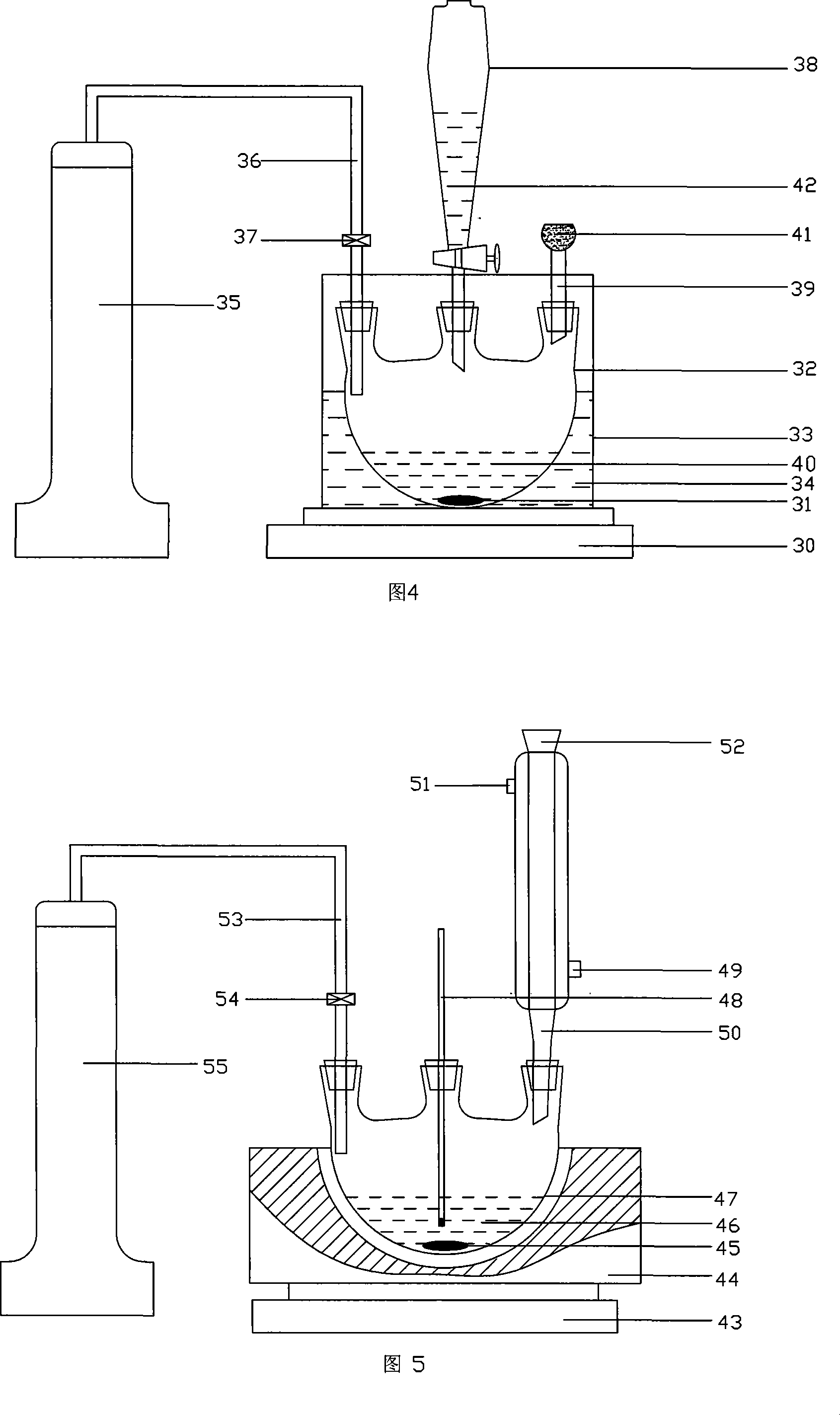
[0158] The present invention will be further described below in conjunction with accompanying drawing:
[0159] As shown in Figure 1, it is a flow chart of the preparation process, which must be carried out in strict accordance with the process parameters and operated in sequence.
[0160] The raw materials of chemical substances required in the preparation of synthetic products are determined within pre-set value ranges in grams, milliliters, centimeters 3 , minute is the unit of measurement, when it is produced industrially, it is measured in kilograms, liters, meters 3 , minute is the unit of measurement.
[0161] The chemical raw materials and auxiliary materials required for the preparation must be strictly selected and their purity controlled. Impurities must not intervene to prevent the formation of by-products and affect the purity and luminescence performance of the product.
[0162] Four-necked flasks, three-necked flasks, beakers, suction filtration flasks, chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com