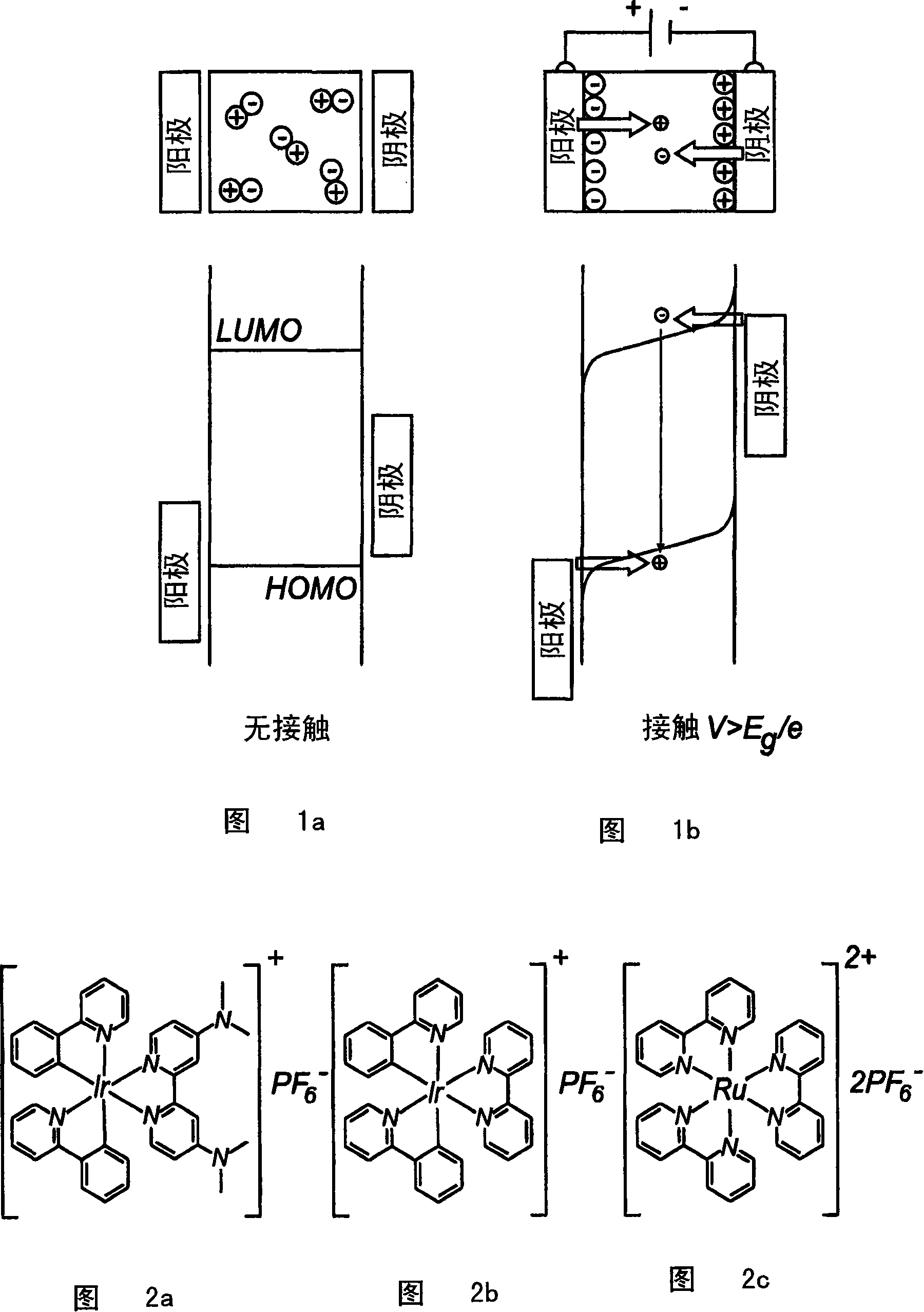
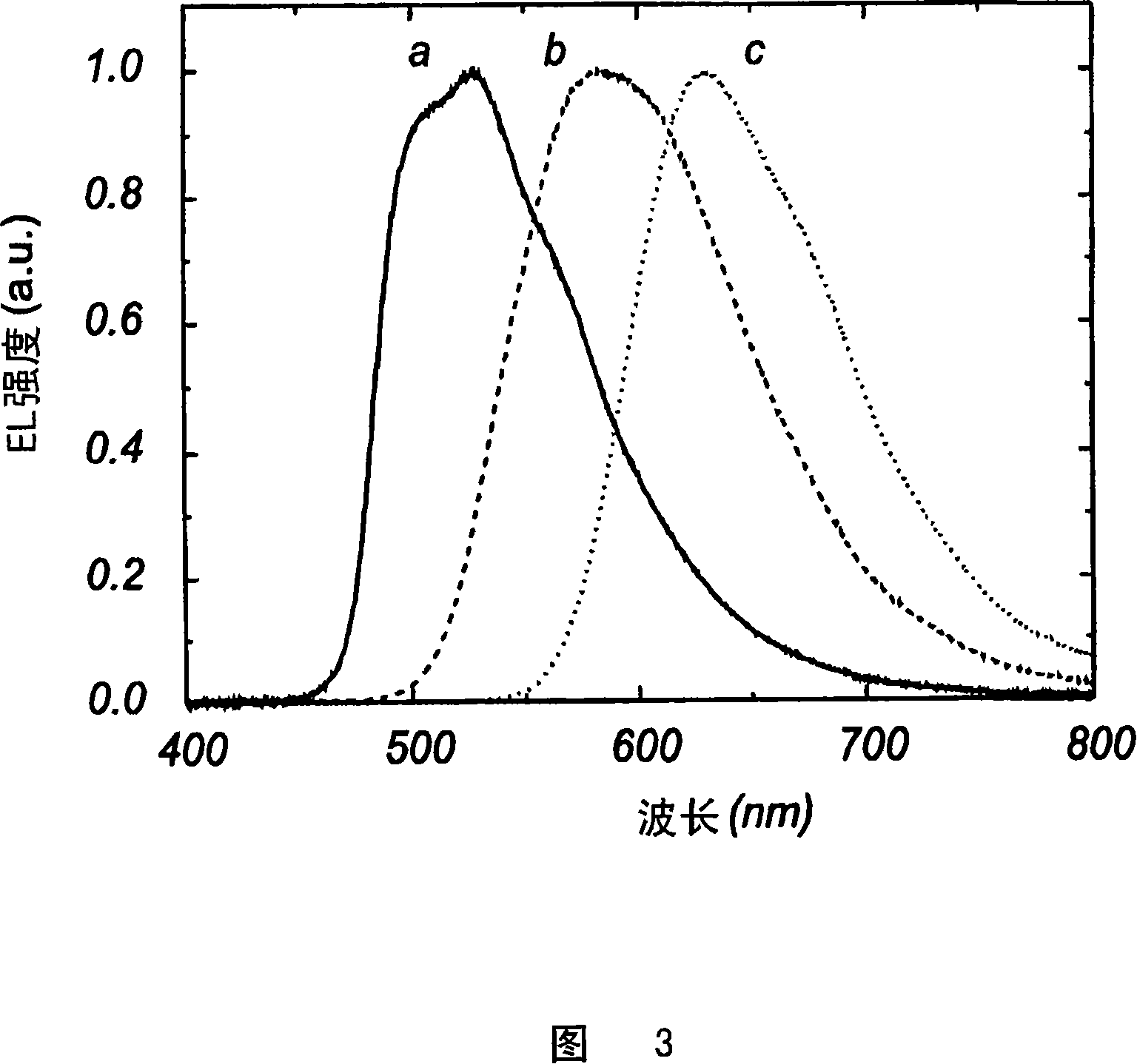
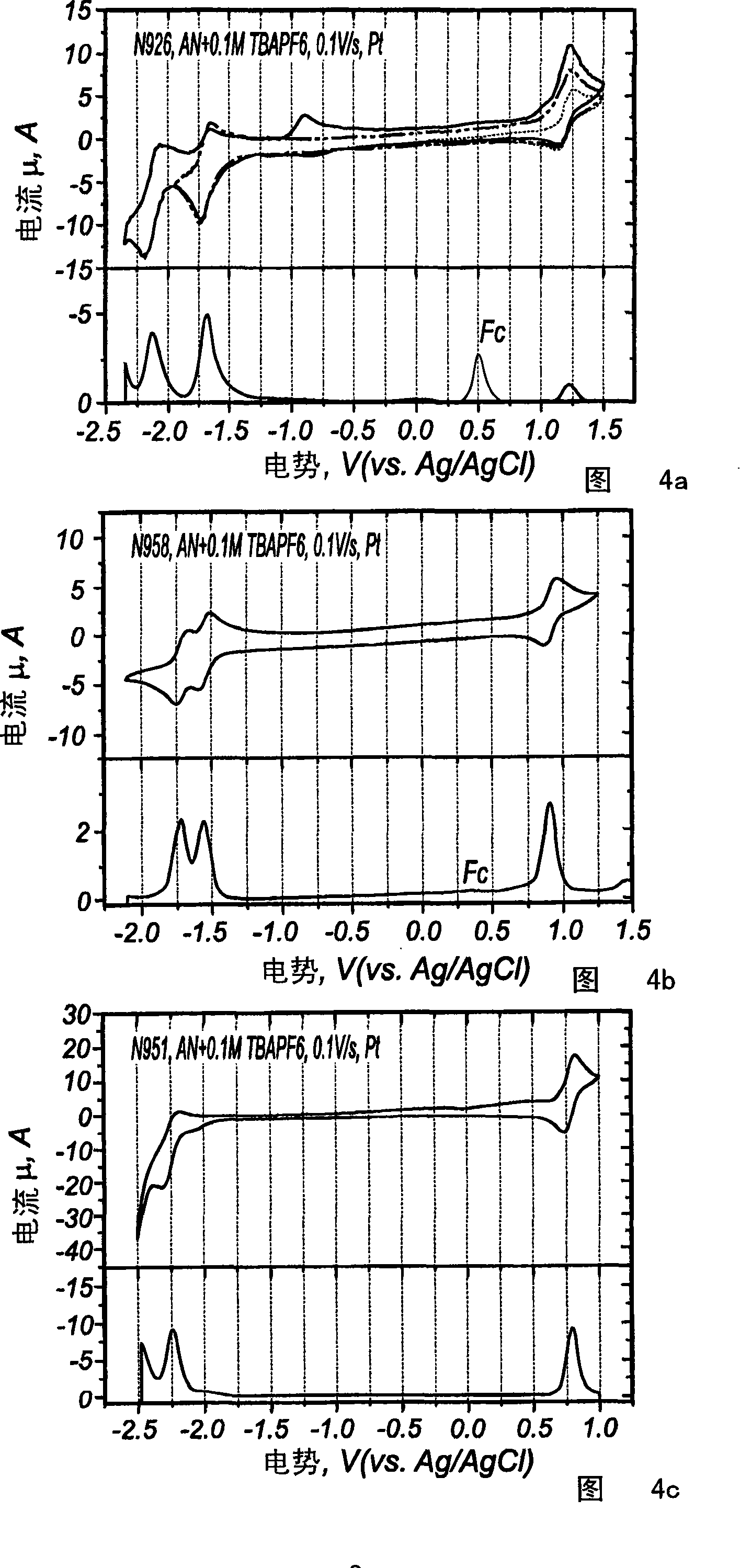
Electro luminescent metal complexes
A technology of metal complexes and compounds, applied in the direction of luminescent materials, indium organic compounds, platinum group organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Synthetic [Ir(ppy) 2 (4,4'dma-bpy)]PF 6 - (Complex 3-1), wherein ppy=2-phenylpyridine and dma-bpy=4,4'-dimethylamino-2,2'-bipyridine
[0189] Dimeric iridium(III) complex [Ir(ppy) 2 (Cl)] 2 (300mg; 0.28mM) was dissolved in 100ml of dichloromethane solvent. To this solution was added 4,4'-dimethylamino-2,2'-bipyridine ligand (176 mg, 0.724 mM). The reaction mixture was refluxed under nitrogen for 3 days (3 hours is sufficient). The solvent dichloromethane was evaporated and the resulting solid was dissolved in 5 mL methanol. Then, by adding a hexafluorophosphate solution in methanol, [Ir(ppy) 2 (4,4'dma-bpy)]PF 6 - (complex 3-1). Yield: 306 mg; 50%.
Embodiment 2
[0191] [Ir(ppy) 2 (4,4'dma-bpy)]PF 6 - (Complex 3-1) Use as an electroluminescent material
[0192] Synthetic [Ir(ppy) 2 (4,4'-dma-bpy)] + (PF 6 - ) (complex 3-1), and 37.1 mg of this complex was dissolved in 1.855 ml of acetonitrile by stirring at 50° C. for 30 minutes. Thus, the concentration of the complex in acetonitrile was 20 mg / ml. The solution was placed in a nitrogen atmosphere glove box where all subsequent handling was performed. Molecular sieves were added to remove traces of water. After 30 min, the solution was filtered and spin-coated on a glass substrate with structured ITO, which had been thoroughly cleaned beforehand with soap, water, isopropanol, ultrasound and UV-ozone. This yielded a uniform film approximately 100 nm thick.
[0193] The film was dried under nitrogen at 100°C for about 1 / 2 hour. In a vacuum chamber, at about 10 -7 millibar, a 100 nm thick silver electrode is evaporated on top at a rate of 0.5 nm / s. This resulted in 4 LEEC devic...
Embodiment 3
[0195] Embodiment 3: complex list
[0196] The 2,3,4,5 positions of the phenyl ring of 2-phenylpyridine are as defined in formula (I).
[0197] Complexes 1 and 2:
[0198]
[0199] 1-1: R 1 = H; R 2 =H 2-1:R 1 = H; R 2 =H
[0200] 1-2:R 1 =CH 3 ; 2 =H 2-2:R 1 =CH 3 ; 2 =H
[0201] 1-3: R 1 =CO 2 CH 3 ; 2 =H 2-3:R 1 =CO 2 CH 3 ; 2 =H
[0202] 1-4: R 1 = 4-dimethylaminostyryl; R 2 =H 2-4:R 1 = 4-dimethylaminostyryl; R 2 =H
[0203] 1-5: R 1 =N(CH 3 ) 2 ; 2 =H 2-5:R 1 =N(CH 3 ) 2 ; 2 =H
[0204] 1-6: R 1 =N(CH 3 ) 2 ; 2 = 2,4-difluoro 2-6: R 1 =N(CH 3 ) 2 ; 2 = 2,4-difluoro
[0205] 1-7: R 1 =N(CH 3 ) 2 ; 2 = 3,5-trifluoro 2-7: R 1 =N(CH 3 ) 2 ; 2 = 3,5-difluoro
[0206] 1-8: R 1 =N(CH 3 ) 2 ; 2 = 3-OCH 3 2-8: R 1 =N(CH 3 ) 2 ; 2 = 3-OCH 3
[0207] 1-9: R 1 = H; R 2 = 2,4-difluoro 2-9: R 1 = H; R 2 = 2,4-difluoro
[0208] Complexes 3 and 4:
[0209]
[0210] 3-1: R 1 = H; R 2 =H 4-1:R 1 = H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com