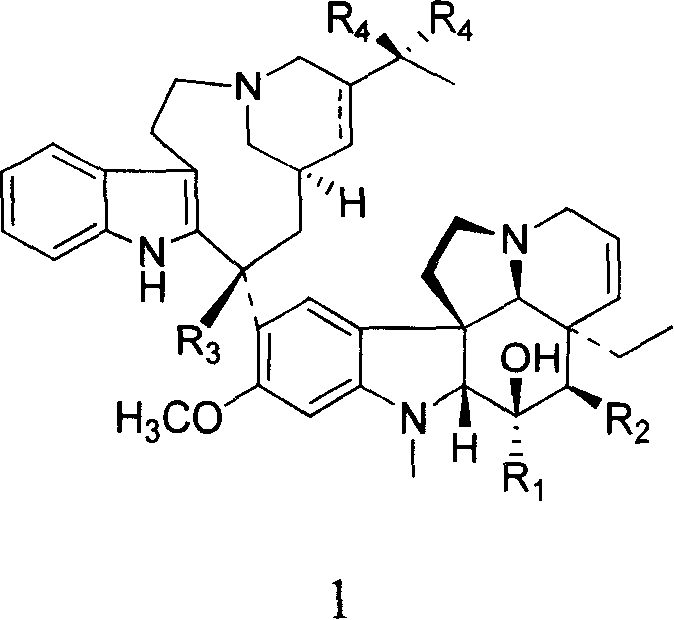
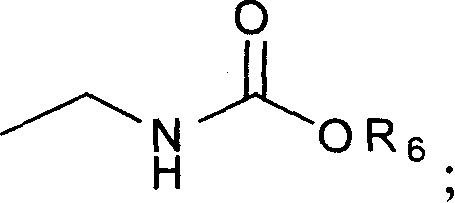
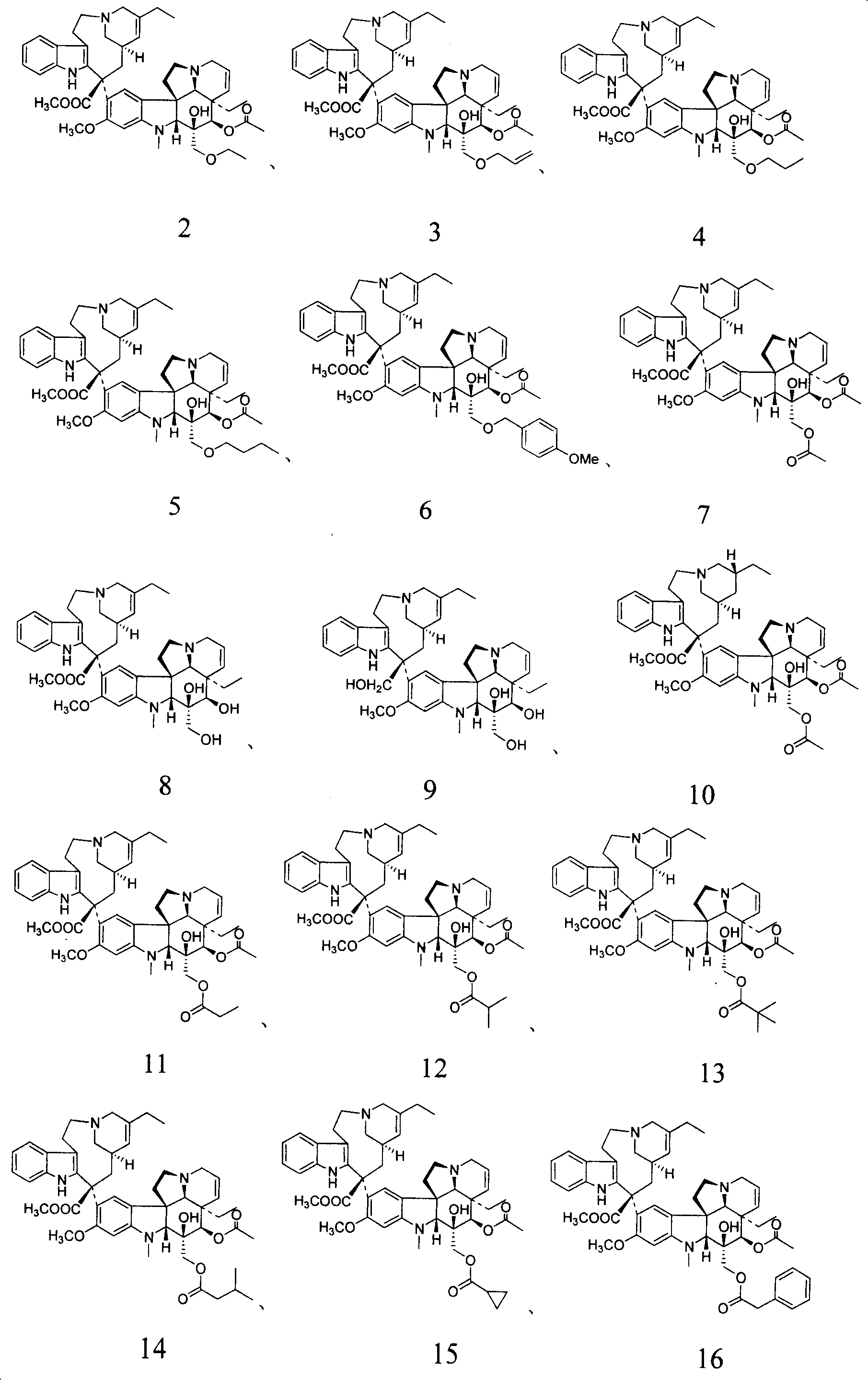
Novel vinblastine derivative, preparation method and use thereof, and medical composition containing the derivative
A technology of vinblastine and its derivatives, applied in the field of medicinal chemistry, which can solve the problems of poor linear correlation of binding activity, inhibition of cell growth, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0070] Preparation of Example 1 Compound A
[0071]
[0072] Under the protection of argon, take 456mg (1mmol) of Wendolin and dissolve it in 20mL of anhydrous tetrahydrofuran, slowly add 230mg (6mmol) of aluminum tetrahydrogen lithium in an ice bath at 0°C, stir at room temperature for 4 hours, add 0.23mL of water to quench Then add 0.23mL of 15% sodium hydroxide and 0.69mL of water successively, stir for 5 minutes, filter with sand star funnel, dry over anhydrous magnesium sulfate, concentrate under reduced pressure, and recrystallize with acetone to obtain white solid compound A, product Rate 85%-90%.
[0073] 1 H NMR (CDCl 3 , 300MHz): δ: 8.73(brs, 1H), 6.82(d, J=8.1Hz, 1H), 6.24(d, J=8.1Hz, 1H), 6.06(s, 1H), 5.80(dd, J= 10.2, 4.8Hz, 1H), 5.60(d, J=10.2Hz, 1H), 3.93(d, J=14.1Hz, 1H), 3.71(s, 3H), 3.54(s, 1H), 2.95(s, 3H), 2.51(s, 1H), 2.43(m, 1H), 2.16(m, 1H), 1.77(m, 1H), 1.30(m, 1H), 0.86(m, 1H), 0.56(t, J = 8.4Hz, 3H);
[0074] 13 C NMR (CDCl 3 , 75MHz): δ: 1...
preparation Embodiment 2
[0076] Preparation of Example 2 Compound B
[0077]
[0078] In a 100mL two-neck round bottom flask, 3.86g (10.00mmol) of compound A was dissolved in 25mL of THF, and 50% NaOH (1g NaOH: 1g H 2O) Stir at 50° C. for half an hour, then add 2.10 g (1.1 eq, 11.00 mmol) of toluene-4-sulfonyl chloride, raise the temperature to 80° C., and stir for 1 h. After the reaction was completed, it was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain an epoxy oily intermediate without purification, and then proceeded to the next step of reaction. In a 250mL round bottom flask, dissolve the oily intermediate in 80mL of methanol and 10mL of water, add 3.25g (5eq) of sodium azide and 1.4g (3eq) of ammonium chloride in sequence, and reflux at 90°C for 24h. After the reaction was completed, it was extracted with ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and subjected to silica gel ...
preparation Embodiment 3
[0081] Preparation of Example 3 Compound C1
[0082]
[0083] Under the protection of argon, take 386mg (1mmol) of compound A and dissolve it in 0mL tetrahydrofuran, add 50% aqueous sodium hydroxide solution (1g sodium hydroxide dissolved in 1g water), stir the reaction at 60°C for 0.5h, then add 0.15mL Bromoethane and 50mg tetra-n-butylammonium iodide, continue to react for 6h, then cool to room temperature, transfer the reaction solution to a separatory funnel, add 50mL water, then extract with dichloromethane (10mL×3), wash over anhydrous Dry over magnesium sulfate, and concentrate under reduced pressure to obtain a concentrate. Under the protection of argon, dissolve the concentrated solution in 1 mL of pyridine, add 1 mL of acetic anhydride, stir the reaction at room temperature for 8 h, then inject 30 mL of ethyl acetate and 10 mL of saturated sodium bicarbonate solution and continue stirring for 2 minutes, remove the water layer, and use Pyridine (20 mL×3) was washe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com