Hole-transporting type blue luminescent material as well as preparation and uses thereof
A technology of hole transport and blue light, which is applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of complicated device preparation, poor carrier injection and transport, and achieve good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
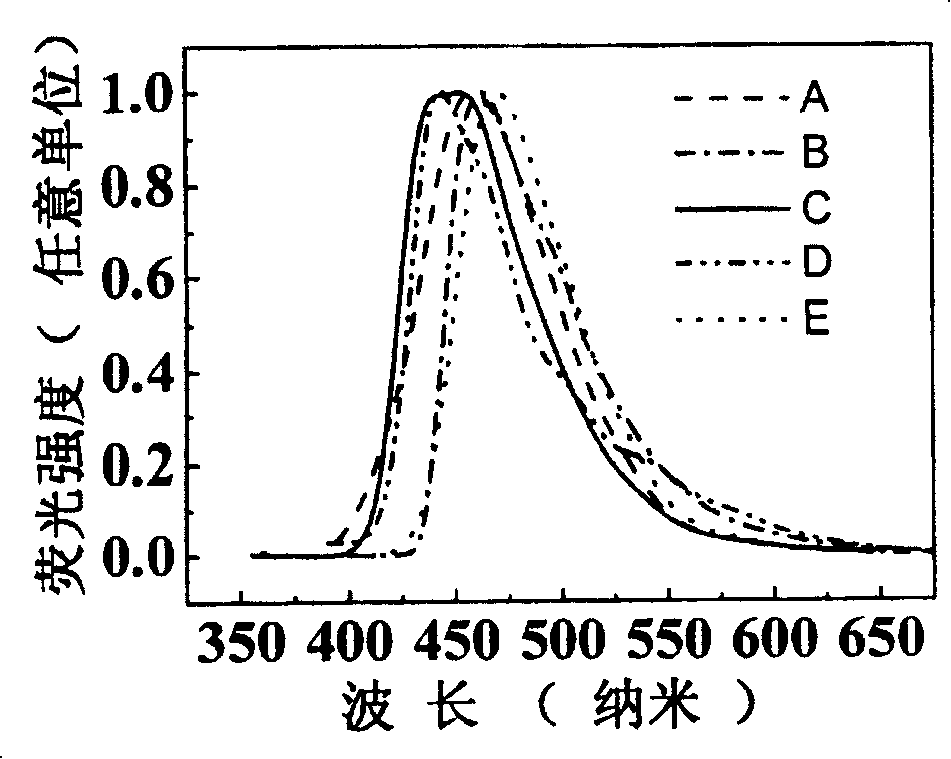
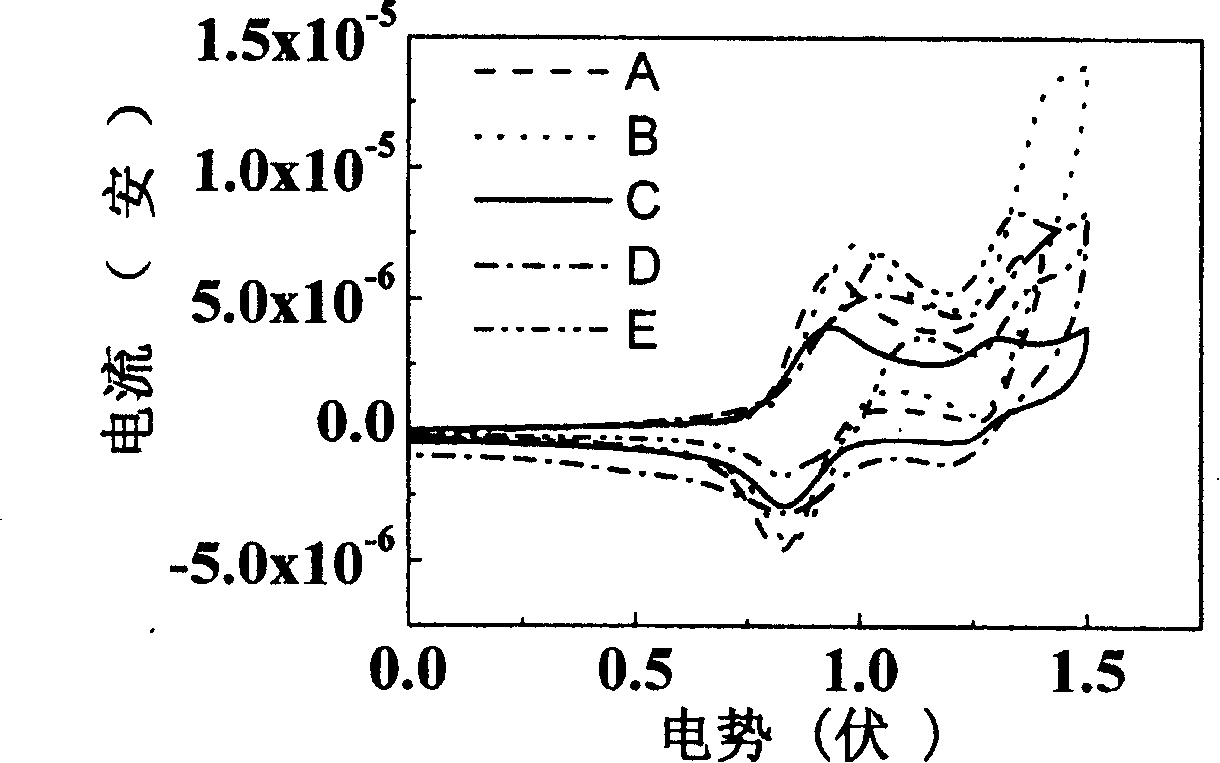
Image
Examples
Embodiment 1
[0064] Synthesis of the precursor 9,9-bis[4-(N,N-dimethylanilino)phenyl]-2-bromofluorene (compound 3).
[0065] 2-Bromofluorenone (4g, 15.4mmol) was mixed with 4,4'-dimethyltriphenylamine (15g, 55mmol), and heated to 120°C under nitrogen atmosphere. After the mixture was completely melted, the catalyst trifluoromethyl Sulfonic acid 1mL, keep heating overnight. After the reaction, dichloromethane was added to dissolve all unreacted raw materials and other impurities, and the white solid obtained by filtration was 9,9-bis[4-(N,N-di-p-toluidyl)phenyl]-2-bromofluorene (Formula 3) 11.0 g, yield 90%. Mass spectrum: m / z 786.7 (M + +1); elemental analysis (%) C 53 h 43 BrN 2 : theoretical value C 80.80, H 5.50, N 3.56; found value C 80.05, H 5.64, N 3.57.
Embodiment 2
[0067] Synthesis of the precursor 9,9-bis[4-(N,N-di-p-toluidyl)phenyl]fluorene-2,7-diboronic acid pinacate (compound 2).
[0068] 2,7-dibromofluorenone (4g, 12mmol) was mixed with 4,4'-dimethyltriphenylamine (15g, 55mmol), and heated to 120°C under nitrogen atmosphere. After the mixture was completely melted, the catalyst trifluoro Methanesulfonic acid 0.75mL, kept heating overnight. After the reaction, the crude product was dissolved in dichloromethane, and part of the solvent was removed under reduced pressure, then poured into a large amount of acetone for precipitation, and the white solid obtained after filtration was 9,9-bis[4-(N,N-di-p-methyl Anilino)phenyl]-2,7-dibromofluorene (compound 1) 8.7g, yield 83%.
[0069] Mass spectrum: m / z 864.9 (M + +1); NMR[ 1 H NMR (CDCl 3 , 300MHz)]: δ=7.57(d, 2H), 7.49(s, 2H), 7.47(d, 2H), 7.06-6.92(m, 20H), 6.86(d, 2H), 2.29(s, 12H) ;Elemental analysis (%) C 53 h 42 Br 2 N 2 : theoretical value C 73.45, H 4.88, N 3.23; found v...
Embodiment 3
[0073] Combination bromides of conjugated groups: Synthesis of 4-(10-bromoanthracen-9-yl)-N,N-diphenylaniline (Compound 8).
[0074] 4-Bromotriphenylamine (3.24g, 10mmol) was dissolved in tetrahydrofuran (30mL), cooled to -78°C under a nitrogen atmosphere, added n-butyllithium (4.8mL, 12mmol), kept at low temperature for about 1 hour, and added isopropoxy boronic acid pinacidate (2.45mL, 12mmol), warmed up to room temperature and stirred overnight. After the reaction, the solvent was removed by rotary evaporation, and petroleum ether was used as the eluent. Silica gel column chromatography gave 2.2 g of the white solid product 4-dianilinophenylboronic acid pinacid (compound 7), with a yield of 59%. Mass spectrum: m / z371 (M + +1); elemental analysis (%) C 24 h 26 BNO 2 : Theoretical value C 77.64, H 7.06, N 3.77; found value C 77.42, H 7.18, N 3.75.
[0075] Compound 7 (1.86g, 5mmol), 9,10-dibromoanthracene (5.04g, 15mmol), tetrahydrofuran (100mL), potassium carbonate aque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com