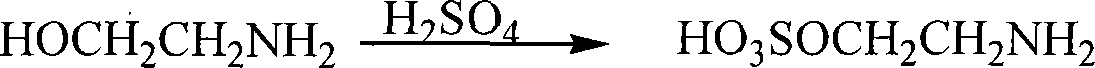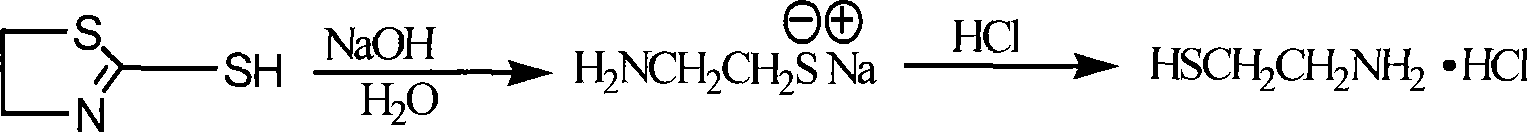Method for preparing cysteamine hydrochloride by basic hydrolysis
A technology of cysteamine hydrochloride and alkali hydrolysis, which is applied in the direction of mercaptan preparation and organic chemistry, can solve the problems of complex operation, difficult discharge, low yield, etc., to reduce production costs, fully utilize resources, and rate-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The specific steps of a method for preparing cysteamine hydrochloride by alkali hydrolysis are as follows:
[0027] (1) Synthesis of 2-aminoethylsulfate
[0028] Under the ice-water bath condition, according to ethanolamine: the molar ratio of sulfuric acid is 1: 1.2, in the three-necked container, first add mass fraction and be 70% ethanolamine solution, then dropwise add mass fraction and be 50% sulfuric acid solution, stir and control while dripping. The temperature of the reaction solution was 10°C. After the dropwise addition was completed, the ice-water bath was removed, and the mixture was stirred at room temperature for 30 minutes. Then, the reaction solution was distilled under reduced pressure until anhydrous was distilled off. The final temperature of the reduced-pressure distillation was 145° C., and 2-aminoethyl sulfate was synthesized as a white solid.
[0029] (2) Synthesis of α-mercaptothiazoline
[0030] After the (1) step was completed, in the white...
Embodiment 2
[0036] A kind of method for preparing cysteamine hydrochloride by alkali hydrolysis is characterized in that:
[0037](1) ethanolamine: the mol ratio of sulfuric acid is 1: 1, and the massfraction of ethanolamine solution is 60%, and the massfraction of sulfuric acid solution is 50%, and dropwise temperature is 20 ℃, stirred at room temperature for 30 minutes, and distilled under reduced pressure The final temperature is 135°C;
[0038] (2) Adding a mass fraction of 20% sodium hydroxide solution, the molar ratio of sodium hydroxide: carbon disulfide: 2-aminoethyl sulfate is 1: 1: 1, and the temperature is raised to 45 ° C. After 2 hours of reaction, the temperature is raised to 55 ° C. ℃;
[0039] (3) The drying temperature is 60°C and the drying time is 4 hours;
[0040] (4) The mass fraction of sodium hydroxide solution is 20%, the mol ratio of α-mercaptothiazoline: sodium hydroxide is 1: 6, atmospheric pressure distillation 3 hours, pH is 3, rinses 4 times with dehydrated...
Embodiment 3
[0042] A kind of method for preparing cysteamine hydrochloride by alkali hydrolysis is characterized in that:
[0043] (1) ethanolamine: the mol ratio of sulfuric acid is 1: 1.2, and the massfraction of ethanolamine solution is 70%, and the massfraction of sulfuric acid solution is 50%, and dropwise temperature is 5 ℃, stirred at room temperature for 30 minutes, and distilled under reduced pressure The final temperature is 145°C;
[0044] (2) Adding a mass fraction of 20% potassium hydroxide solution, the molar ratio of potassium hydroxide: carbon disulfide: 2-aminoethyl sulfate is 1: 1.6: 1, and the temperature is raised to 45° C. After 2 hours of reaction, the temperature is raised to 55 ℃;
[0045] (3) The drying temperature is 60°C and the drying time is 4 hours;
[0046] (4) The mass fraction of sodium hydroxide solution is 20%, the mol ratio of α-mercaptothiazoline: sodium hydroxide is 1: 4, atmospheric pressure distillation 3 hours, pH is 2, wash 4 times with dehydrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com