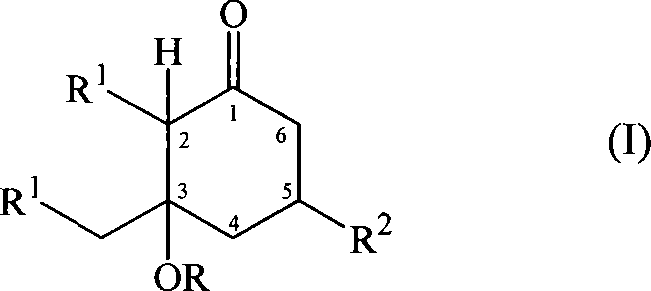
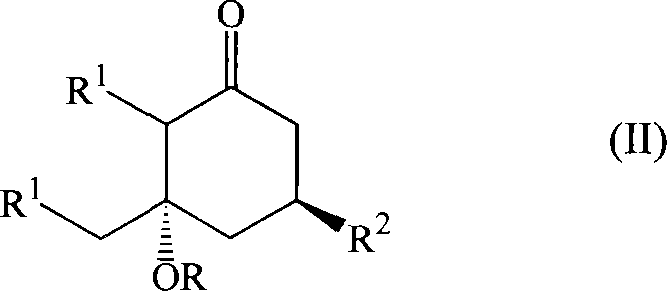
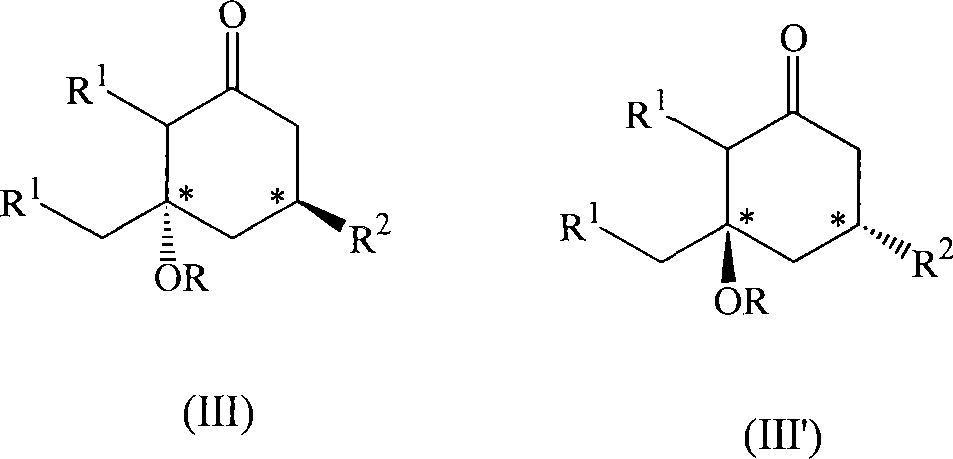
Substituted cyclohexanones
A cyclic, mandatory technique used in the preparation of organic compounds, compounds of elements of group 4/14 of the periodic table, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Preparation of (-)-(1R,11S,14R)-1-Hydroxy-14-methyl-bicyclo[9.4.0]pentadecane-12- Ketone (13) and (-)-(1R,11R,14R)-1-hydroxy-14-methyl-bicyclo[9.4.0]pentadecane-12- Ketones (14)
[0143] Catalyst preparation: CH with TiCl4 (9.50g (5.52ml); 50mmol) 2 Cl 2 (50ml) (temperature 30~40 ℃) solution handles the CH of freshly distilled (+)-N-isopropylephedrine (10.35g, 50.0mmol) within 5 minutes 2 Cl 2 (150ml) solution. After joining, at N 2 The solvent was distilled down. The residue was dried under vacuum (0.01 mbar): 21.77 g.
[0144] Aldol reaction: The catalyst obtained above (20.71 g; max. 47.6 mmol; 0.80 equivalents) was poured into a 250 ml container containing NMP (57 ml) while stirring. The temperature was brought to 34°C. After 5 minutes, solid 3-methyl-1,5-cyclopentadecanedione (15) (14.98 g, 59.4 mmol) was added to the stirred dark brown solution. After 5 minutes, use H 2 O (89.1 mg; 4.95 mmol) of TMEDA (6.67 g, 8.54 ml, 57.5 mmol) and the solution wa...
Embodiment 2
[0156] Preparation of (1RS, 11RS, 14RS)-1-hydroxy-14-methyl-bicyclo[9.4.0]pentadecane-12- Ketones (16)
[0157] with ZrCl 3 A solution of OPr (36%, in AcOEt) (65.25g, 91.6mmol) was treated with CH 2 Cl 2 (210ml) solution. After 5 minutes, use NBu 3 (19.43g, 25.0ml, 105mmol) the light yellow solution was treated at -10~0°C. After 15 minutes, the reaction mixture was poured into water and extracted with ether. Washing (H 2 O, saturated NaHCO 3 , NaCl), dry (Na 2 SO 4 ) and evaporate the organic phase. Crystallization from heptane (345ml) yielded 11.93g of (16) (yield: 79%) and 2.80g of mother liquor, from which an additional 1.39g (yield: 9%) of (16) was recovered. The spectrum is the same as that of (14).
Embodiment 3
[0159] Preparation of (+)-(S)-14-methylbicyclo[9.4.0]pentadeca-1(11)-en-12-one (17)
[0160] use me 3 N·HCl (1.89 g; 19.80 mmol) treatment of (13) / (14) (9.97 g; 39.56 mmol) (obtained by repeating Example 1 after one chromatography) 2 Cl 2 (300ml) solution. At -15°C, with NEt 3 (15.98 g, 158.2 mmol) and the stirred mixture was continuously treated dropwise with MsCl (13.58 g, 118.7 mmol). The reaction mixture was stirred at 0°C for 1 hour and at 25°C for 15 hours. It was then poured into 5% HCl and extracted with ether. Washing (H 2 O, saturated NaHCO 3 , NaCl), dry (Na 2 SO 4 ) and evaporation of the organic phase gave a crude oil. The oil was heated at 80°C in toluene (60ml) and DBU (9.02g, 59.34mmol) for 1 hour, cooled and purified etc. as described above (5% HCl and extraction). Bulb-to-bulb distillation (oven temperature 100-150° C. / 0.01 mb) afforded 8.72 g (yield: 94%) of (17) (29% ee).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com