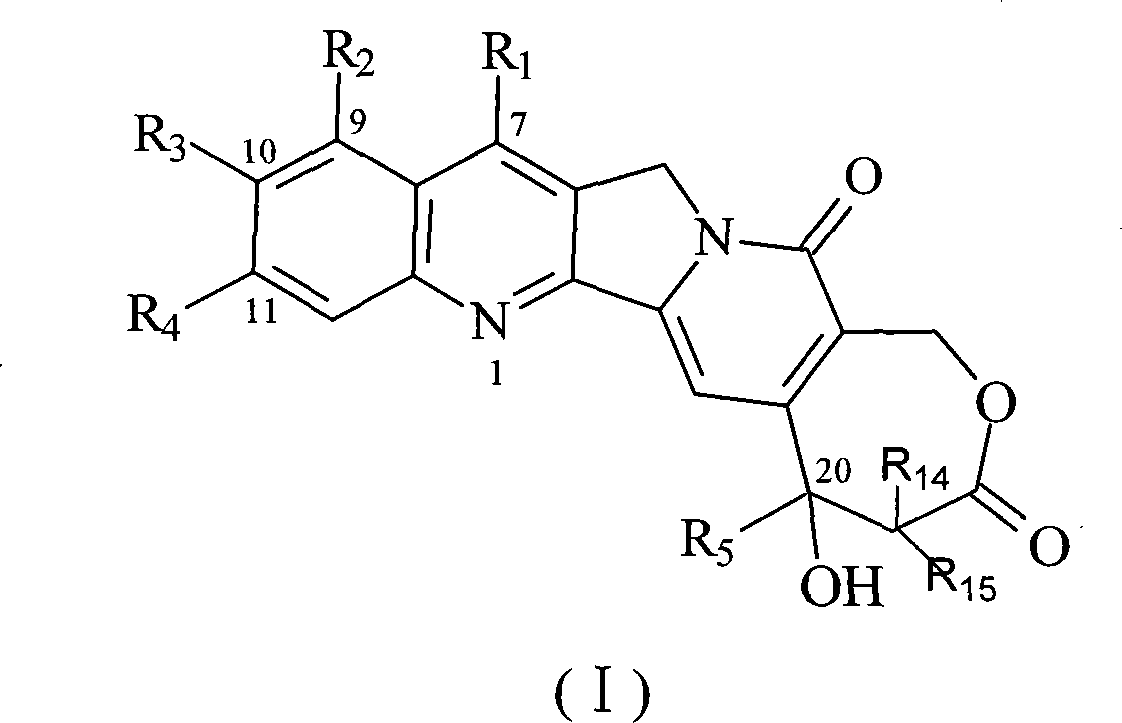
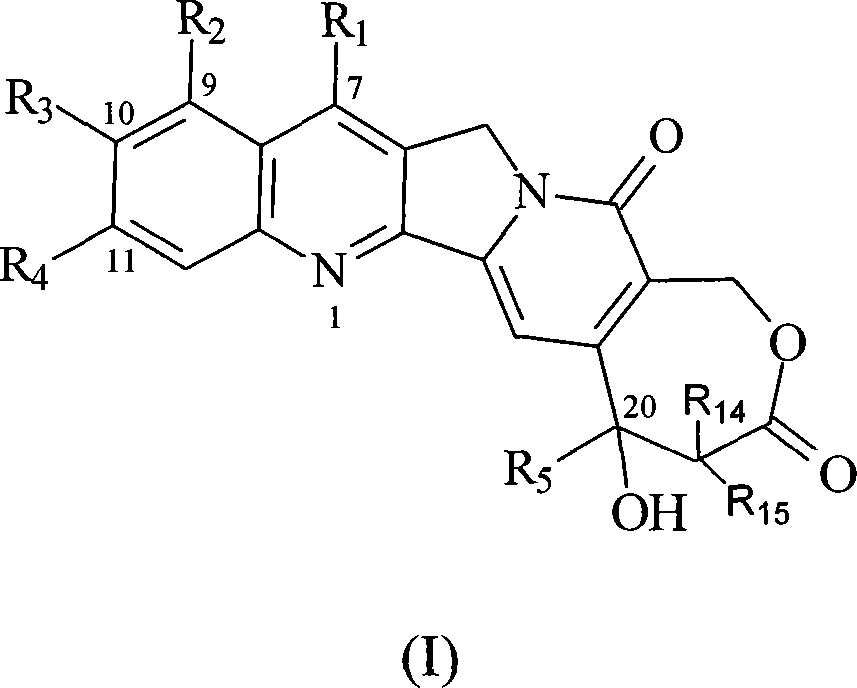
10-substitution homocamptothecin compounds and uses
A technology of homocamptothecin and compound, applied in the field of medicine, can solve the problems of large toxic and side effects, poor water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The synthesis of embodiment 1 7-methyl-10-hydroxyl homocamptothecin
[0064] 0.50g of 9-ethyl-9-hydroxyl-2,3,8,9-tetrahydro-5H-6-oxa-3a-aza-cycloheptane-1,4,7-trione and 0.50g 5-Hydroxy-2-aminoketone was dissolved in 500 mL of toluene, refluxed to separate water, after 30 minutes, 0.10 g of p-toluenesulfonic acid was added, the reflux was continued for 4 h, the solvent was evaporated, washed with acetone and methanol, and 0.5 g of yellow solid 7- Methyl-10-hydroxyhomamptothecin (70.7%).
[0065] 1 HNMR(DMSO), δ: 0.87(t, 3H), 1.86(q, 2H), 2.76(s, 3H), 3.06(d, 1H), 3.49(d, 1H), 5.28(s, 2H), 5.39 (d, 1H), 5.53(d, 1H), 6.03(s, 1H), 7.42(s, 1H), 7.72(t, 1H), 7.87(t, 1H), 8.15(q, 2H), 8.69( s, 1H), 10.32 (s, 1H).
Embodiment 2
[0066] Example 2 Synthesis of 7-methyl-10-tert-butoxycarbonyl phenylalanine ester-based homocamptothecin
[0067] Dissolve 50mg of tert-butoxycarbonylphenylalanine and 50mg of N, N cyclohexyl carboximide in 10ml of dichloromethane, stir at room temperature for 10min, add 50mg of p-dimethylaminopyridine, continue stirring for 10min, then add 50mg of 7-methyl- 10-Hydroxyhomocamptothecin, after reacting for 6 hours, evaporate the solvent to dryness, add 10ml ether, filter, and the filter cake is purified on a silica gel chromatographic column (eluent: CH 2 Cl 2 / CH 3 OH 100:5), to obtain 21 mg of yellow solid 7-methyl-10-tert-butoxycarbonylphenylalanine ester homocamptothecin (25.8%).
[0068] 1 HNMR(DMSO), δ: 0.87(t, 3H), 1.40(s, 9H), 1.86(q, 2H), 2.73(s, 3H), 3.14(d, 2H), 3.27(d, 2H), 4.50 (q, 1H), 5.30(s, 2H), 5.47(d, 2H), 6.04(s, 1H), 7.29(s, 1H), 7.37-7.40(m, 5H), 7.51(d, 1H), 7.69 (d, 1H), 7.72 (s, 1H), 8.20 (d, 1H).
Embodiment 3
[0069] Example 3 Synthesis of 7-methyl-10-tert-butoxycarbonyl glycinate-based homocamptothecin
[0070] According to the method of Example 2, tert-butoxycarbonylglycine was used instead of tert-butoxycarbonylphenylalanine to obtain 22 mg of yellow solid 7-methyl-10-tert-butoxycarbonylglycinyl homocamptothecin (31.4%).
[0071] 1 HNMR(DMSO), δ: 0.87(t, 3H), 1.42(s, 9H), 1.87(q, 2H), 2.75(s, 3H), 3.27(d, 2H), 4.08(d, 2H), 5.30 (s, 2H), 5.47(d, 2H), 6.04(s, 1H), 7.40(s, 1H), 7.49(t, 1H), 7.65(d, 1H), 7.97(s, 1H), 8.20( d, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com