Immune bovine colostrum and method for preparing bifidobacteria composite tiny capsule and pill
A bifidobacterium and colostrum technology, applied in the direction of medical preparations containing active ingredients, sugar-coated pills, pill delivery, etc., can solve the problems of reducing the activity, quantity and potency of immune bovine colostrum and bifidobacteria, and achieve Good release effect in the intestinal tract, strong acid resistance and anti-digestive enzyme properties, high immunity and micro-ecological regulation dual activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of bifidobacterium fermented immune bovine colostrum
[0028] 1. Preparation of immune bovine colostrum
[0029] 1. Antigen preparation
[0030] Shigella flexneri (Shigella flexneri, purchased from the Microbiology Laboratory of the First Clinical Hospital of Jilin University, conventional strains) was enriched and cultured at a shaking table temperature of 37°C and a rotational speed of 200rpm for 18-20 hours; the culture solution was collected and Add 0.4% formaldehyde at a volume concentration, inactivate at 37°C for 24 hours, and perform miscellaneous inspection. If there is no viable bacteria, use a McFarland tube to measure the bacterial concentration. Adjust the cell concentration to 1.0×10 with sterile normal saline 11 cfu / mL to make water-based seedlings, and add an equal volume of No. 10 white oil to the water-based seedlings, stir and emulsify to make oil-adjuvanted seedlings; both water-based seedlings and oil-adjuvanted seedlings a...
Embodiment 2
[0051] Example 2: Preparation of immunized bovine colostrum and bifidobacterium composite freeze-dried powder
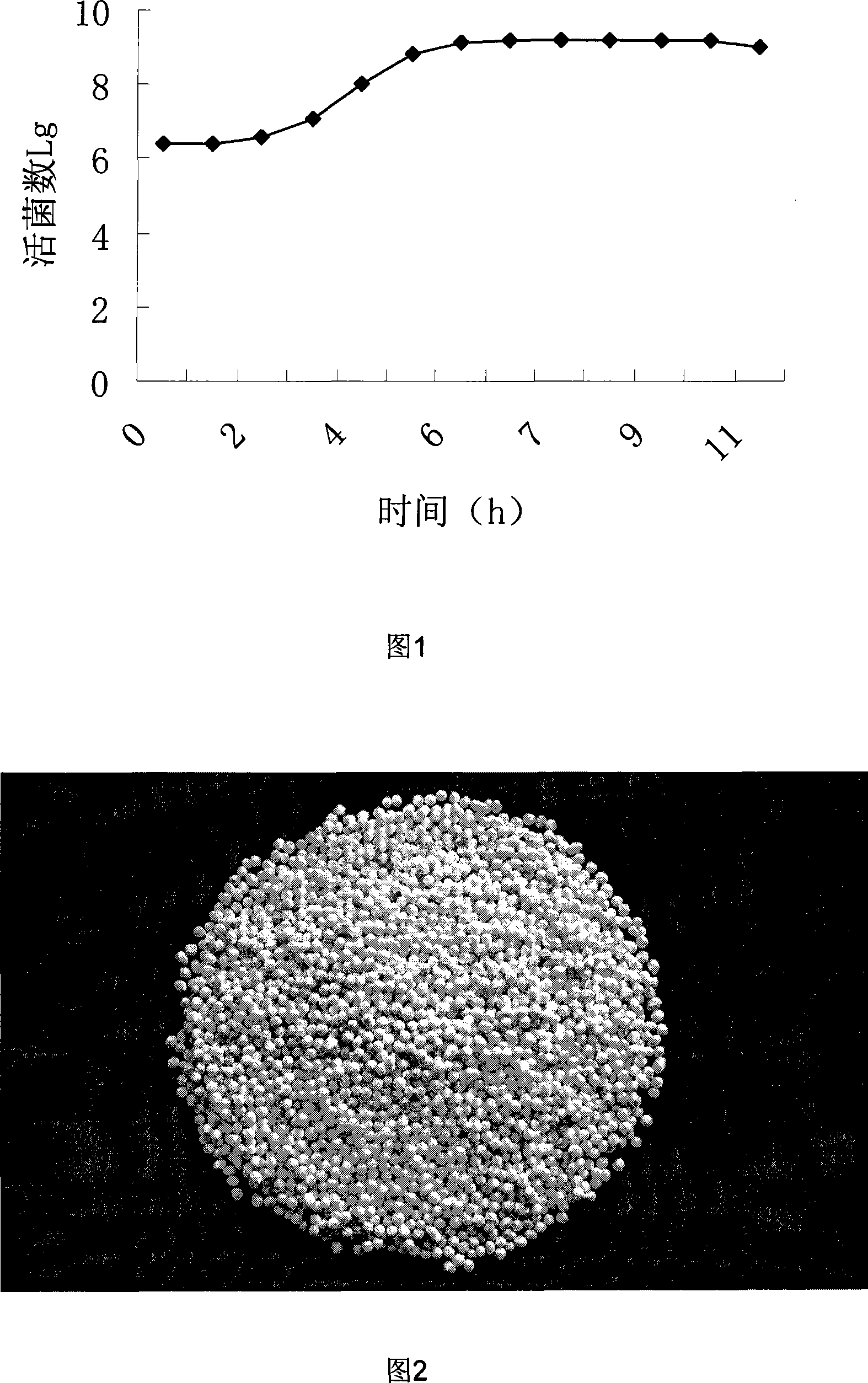
[0052] Add 1% mass concentration of sucrose and 4% mass concentration of mannitol in bifidobacterium fermented immunized bovine colostrum as lyoprotectant. The temperature of the cold trap is -45°C, and the thickness of the feed liquid is 10mm. First, it is pre-frozen at -30°C for 2 hours; The temperature was controlled below 20°C, and the freeze-drying time was 10 hours. The number of viable bacteria in the compound freeze-dried powder was 1.24×10 10 cfu / g dry powder, immunoglobulin titer 2 9 .
Embodiment 3
[0053] Example 3: Preparation of immune bovine colostrum and bifidobacterium composite microcapsules
[0054] 1. Production of mother core
[0055] The mother core is made of sucrose powder and soluble starch with a mass ratio of 7:3. The working parameters of the mother core granulator are: host speed regulation: 80rpm, spray speed regulation: 110rpm, blast temperature: 27.1°C.
[0056] 2. Drying of the mother core
[0057] After the master core is preliminarily shaped and dried, the unqualified master batches are removed, and the drying temperature is 40°C, the relative humidity of the air is ≤20%, and the drying time is 4 hours. The average weight of the mother core is 0.226mg, and the diameter is 0.3-0.5mm.
[0058] 3. Preparation of immunized bovine colostrum and bifidobacterium composite freeze-dried powder particles
[0059] The immune bovine colostrum and bifidobacterium composite freeze-dried powder were coated on the mother core by air suspension granulation metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viable count | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com