Method for preparing doping titanium dioxide nano crystal
A technology of nanocrystals and titanium dioxide, applied in the direction of titanium dioxide, titanium oxide/hydroxide, etc., can solve the problems that TiO2 cannot withstand high-temperature heat treatment materials and affect the photocatalytic performance of TiO2, and achieve excellent photocatalysis, simple preparation method, small size evenly distributed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1) Mix butyl titanate, vanadyl acetylacetonate and ethanol in a container at a molar ratio of 1:0.15:6 and stir for 30 minutes to obtain liquid A;
[0017] 2) Adjust the pH value of the water to 5 with nitric acid to obtain liquid B, the ratio of the moles of water to the sum of the moles of butyl titanate and vanadyl acetylacetonate is 40;
[0018] 3) Add liquid B into liquid A drop by drop, and stir until a stable sol is formed;
[0019] 4) Pour the sol into a container with a large bottom area, place it to dry under normal temperature and ventilated conditions, remove water and organic solvents, and obtain doped titanium dioxide nanocrystals.
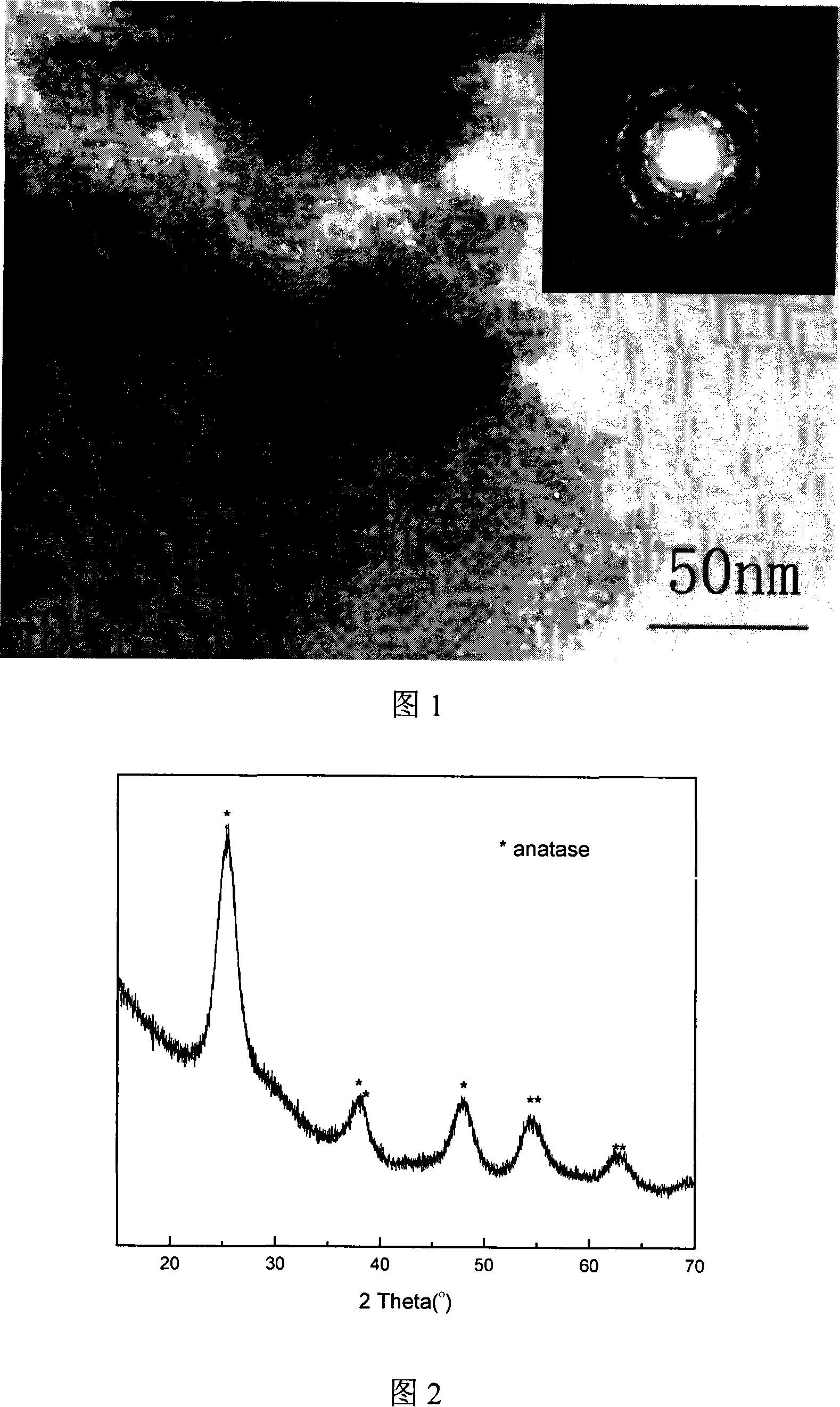
[0020] The transmission electron microscope photo of the prepared doped titanium dioxide crystal is shown in Figure 1, and the upper right corner of the photo is the diffraction ring of the crystal. The X-ray diffraction pattern of the doped titanium dioxide crystal is shown in Figure 2. It can be seen from Fig. 1 and Fig. 2...
Embodiment 2
[0022] 1) Mix isopropyl titanate, niobium hydroxide and ethanol in a container at a molar ratio of 1:0.001:10 and stir for 15 minutes to obtain liquid A;
[0023] 2) Adjust the pH value of the water to 3 with nitric acid to obtain liquid B, the ratio of the moles of water to the sum of the moles of isopropyl titanate and niobium hydroxide is 100;
[0024] 3) Add liquid B into liquid A drop by drop, and stir until a stable sol is formed;
[0025] 4) Pour the sol into a container with a large bottom area, place it to dry under normal temperature and ventilated conditions, remove water and organic solvents, and obtain doped titanium dioxide nanocrystals.
[0026] The obtained anatase phase titanium dioxide crystals have a size of 7 nanometers and a uniform size distribution.
Embodiment 3
[0028] 1) Mix titanium chloride, vanadyl acetylacetonate and propanol in a container at a molar ratio of 1:0.3:18 and stir for 30 minutes to obtain liquid A;
[0029] 2) adjust the pH value of the water to 2 with hydrochloric acid to obtain liquid B, the ratio of the moles of water to the sum of the moles of titanium chloride and vanadyl acetylacetonate is 150;
[0030] 3) Add liquid B into liquid A drop by drop, and stir until a stable sol is formed;
[0031] 4) Pour the sol into a container with a large bottom area, place it to dry under normal temperature and ventilated conditions, remove water and organic solvents, and obtain doped titanium dioxide nanocrystals.
[0032] The obtained anatase phase titanium dioxide crystals have a size of 6 nanometers and uniform size distribution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com