Load type catalyst for olefinic polymerization and method for producing the same
A supported catalyst, olefin polymerization technology, applied in the production of bulk chemicals, etc., can solve the problem of narrow molecular mass distribution of polypropylene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
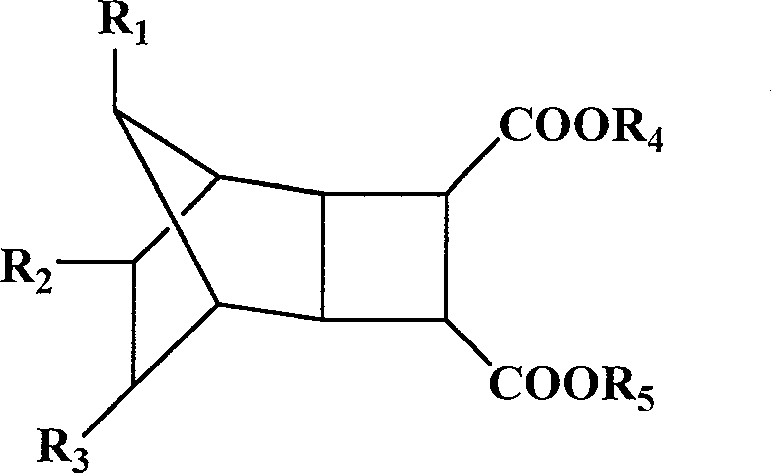
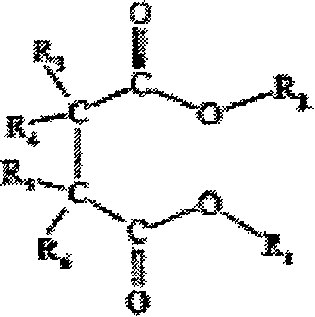
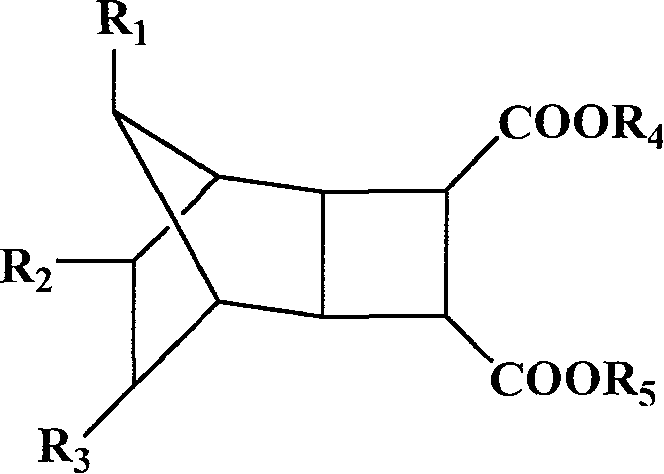
[0083] Example 1 Synthesis of bicyclo[2,2,1]-2,3-α,β ethyl succinate
[0084] In 150 mL of dichloromethane, add 1.17 g of maleic anhydride, 5.00 g of norbornene, and 0.58 g of benzophenone, and irradiate with ultraviolet light for 12 hours while stirring. After removing the solvent, add 30mL ethanol, 3mL H 2 SO 4 , heated to reflux at 100°C for 4 hours. After removing the solvent, extract with 3×50 mL ether, wash with saturated sodium bicarbonate solution, and dry over anhydrous magnesium sulfate. After removing ether, distill under reduced pressure at 104°C (10 mmHg) to obtain 1.51 g of ethyl bicyclo[2,2,1]-2,3-α,β-succinate as a colorless liquid.
[0085] through 1 H NMR (CDCl 3 , 300MHz) Analysis result: δ1.07(m, 3H, CH 2 ); δ1.30(t, 3H, CH 3 ); δ1.50(m, 5H, CH 2 , CH); δ2.18(d, 2H, CH); δ3.38(m, 2H, CH); δ4.24(q, 2H, CH 2 ).
Embodiment 2
[0086] Example 2 Synthesis of bicyclo[2,2,1]-2,3-alpha,beta methyl succinate
[0087] Except that methanol was used instead of ethanol in the synthesis, all the other steps were the same as in Example 1. At 102°C (10mmHg), 1.38g of methyl bicyclo[2,2,1]-2,3-α,βsuccinate was obtained as a colorless liquid.
[0088] through 1 H NMR (CDCl 3 , 300MHz) analysis results: δ1.30-1.60 (m, 8H, 2CH, 6CH 2 ); δ1.73(q, 1H, CH); δ2.04(q, 1H, CH); δ3.10(t, 1H, CH); δ3.67(s, 3H, CH 3 ).
Embodiment 3
[0089] Example 3 Synthesis of diethyl bicyclo[2,2,1]-2,3-alpha,beta succinate
[0090] In 150 mL of dichloromethane, add 1.17 g of maleic anhydride, 5.00 g of norbornene, and 0.58 g of benzophenone, and irradiate with ultraviolet light for 12 hours while stirring. After the solvent was removed, 30 mL of ethanol and 0.1 g of p-toluenesulfonic acid were added, and heated to reflux at 100° C. for 48 hours. After removing the solvent, extract with 3×50 mL ether, wash with saturated sodium bicarbonate solution, and dry over anhydrous magnesium sulfate. After filtering to remove the ether, 1.67 g of diethyl dicyclo[2,2,1]-2,3-α,β-succinate was obtained as colorless viscous bicyclo[2,2,1]-2,3-α,β.
[0091] through 1 H NMR (CDCl 3 , 300MHz) analysis results: δ1.30-1.55 (m, 12H, 6CH 2 , 6CH 3 ); δ2.04(m, 2H, CH); δ3.14(m, 2H, CH); δ4.12(m, 4H, CH 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com