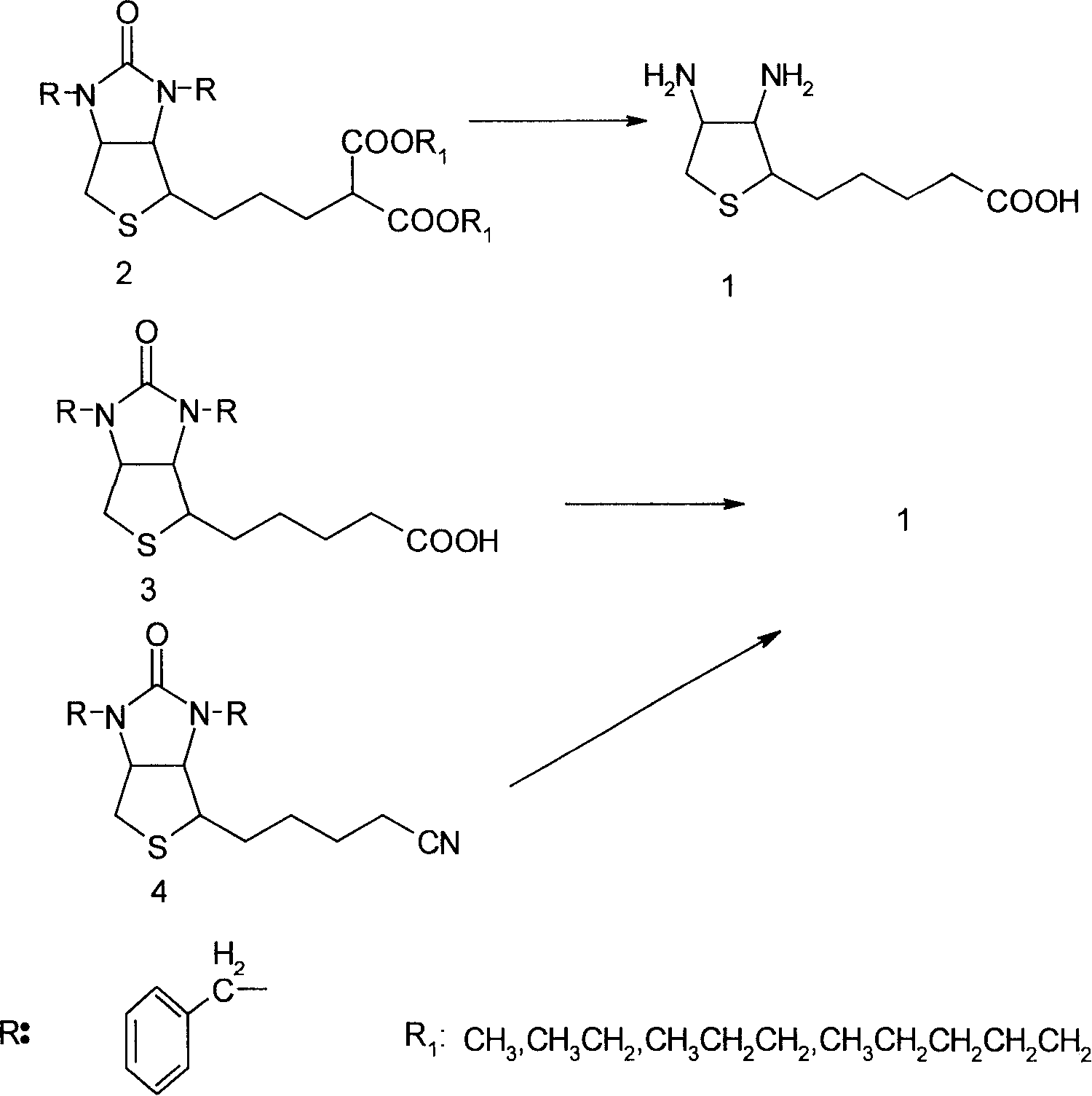
Method for synthesizing (2S,3S,4S)-5-(3,4-diamino-tetrahydrothiophene-2-base) valeric acid
A technique for the synthesis of tetrahydrothiophene, which is applied in the direction of organic chemistry, can solve the problems of affecting the quality of d-biotin, low material yield, long reaction time, etc., and achieve easy reaction end point, high product yield and content, The effect of short reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1 synthesizes diamino acid 1 with diethyl malonate condensate 2
[0012] In the 2000ml three-necked flask, drop into 131g (0.25mol) diethyl malonate condensate 2, 1310g of 52% hydrobromic acid, stir, distillation is heated up to 90 DEG C, is incubated 1 hour, makes bromoethane and decarboxylation reaction complete, change Reflux and continue to heat up at 125-126 ° C, keep warm for 3 hours, TLC (thin layer spot plate analysis) detection (developing agent: toluene: glacial acetic acid: methanol = 10: 10: 3) shows that the reaction system is dibenzyl biotin content 0.8%, 5% biotin and hydrobromide of diamino acid 1, lower the temperature to 110°C, slowly add 400g of glacial acetic acid dropwise, reflux at this temperature for about 2 hours to remove the remaining small amount of monobenzyl biotin, d-bio The prime debenzylation ring is completely opened. Vacuum recover hydrobromic acid and glacial acetic acid, entrain the acid three times with 100ml of water ea...
Embodiment 2
[0018] Embodiment 2 synthesizes diamino acid 1 with cyano compound 4
[0019] In 1000ml there-necked flask, drop into 40.5g (0.1mol) cyano compound 4, 500g of 50% hydrobromic acid, stir, heat up at 125-126 ℃, keep warm for 6 hours, TLC detects (developing agent: ethyl acetate: ethanol=1 : 1) shows that the reaction system is basically 0.6% of dibenzyl biotin content, biotin 7% hydrobromide of diamino acid 1, lower the temperature to 110°C, slowly add 100g of glacial acetic acid dropwise, and reflux at this temperature for about 2 Hours, the remaining small amount of monobenzyl biotin and d-biotin are completely debenzylated and ring-opened. Vacuum recover hydrobromic acid and glacial acetic acid, entrain with 150ml of water three times, add 500ml of absolute ethanol to reflux for 1 hour, cool in an ice-water bath at 0°C, slowly add 8.4g of lithium hydroxide to neutralize, precipitate white solid crystals, filter, filter cake Rinse three times with absolute ethanol, vacuum dry...
Embodiment 3
[0020] Embodiment 3 synthesizes diamino acid 1 with bisbenzyl biotin 3
[0021] In a 2000ml three-necked flask, drop 85g (0.2mol) of bisbenzyl biotin 3, 800g of 51% hydrobromic acid, stir, heat up at 125-126°C, keep warm for 5 hours, TLC detection (developing agent: ethyl acetate: ethanol = 1:1) shows that the reaction system is basically 1% of bisbenzyl biotin, 10% of biotin and hydrobromide of diamino acid 1, lower the temperature to 110°C, slowly add 200g of glacial acetic acid dropwise, and reflux at this temperature In about 2 hours, the remaining small amount of monobenzyl biotin and d-biotin are completely debenzylated and ring-opened. Vacuum recover hydrobromic acid and glacial acetic acid, entrain with 200ml of water three times, add 1000ml of absolute ethanol to reflux for 1 hour, cool in an ice-water bath at 0°C, slowly add 16.8g of lithium hydroxide to neutralize, precipitate white solid crystals, filter, and filter cake Rinse three times with absolute ethanol, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com