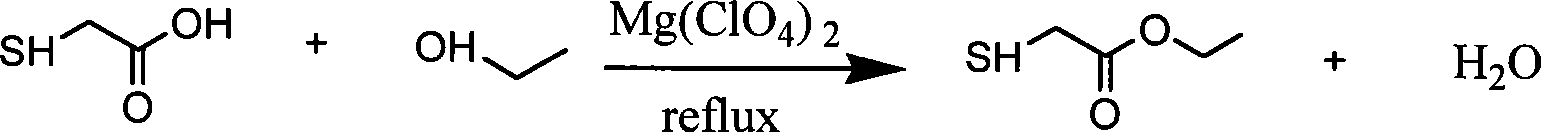Method for producing mercaptoacetic acid ethyl ester
A technology of ethyl mercaptoacetate and ethyl acetate, applied in the field of organic chemical synthesis, can solve the problems of complicated process and high cost, and achieve the effects of simple process, improved yield and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: in the round bottom flask of 100ml, add the mercaptoacetic acid of 27.6g (300mmol), the ethanol of 20.7g (450mmol), the cyclohexane of 16ml, the magnesium perchlorate of 3.34g (15mmol), under stirring Heat to reflux, water will continue to come out from the water separator, stop the reaction until no more water is separated out; distill off excess alcohol and cyclohexane under normal pressure, then add 10ml of distilled water, and then extract with diethyl ether (10ml×3) , organic phase with MgSO 4 Dry, filter, remove ether, then distill under reduced pressure at 80-82°C / 1.3KPa-1.35KPa, collect fractions to obtain ethyl thioglycolate with a yield of 90%. The characterization data of gained ethyl thioglycolate product are as follows:
[0016] IR (KBr): 3433, 2922, 2854, 1740, 1596, 1461, 1423, 1251, 1118, 1040, 801, 671, 621.
[0017] 1 HNMR (400MHz, CDCl 3 , δppm): 4.23-4.17 (m, 2H), 3.26-3.22 (m, 2H), 2.02-1.98 (m, 1H), 1.31-1.23 (m, 3H).
[0018] 1...
Embodiment 2
[0019] Embodiment 2: in the round bottom flask of 100ml, add the mercaptoacetic acid of 27.6g (300mmol), the ethanol of 27.6g (600mmol), the cyclohexane of 16ml, the magnesium perchlorate of 3.34g (15mmol), under stirring Heat to reflux, water will continue to come out from the water separator, stop the reaction when no more water is separated, distill off excess alcohol and cyclohexane under normal pressure, then add 10ml of distilled water, and then extract with diethyl ether (10ml×3) , organic phase with MgSO 4 Dry, filter, remove ether, then distill under reduced pressure at 80-82°C / 1.3KPa-1.35KPa, collect fractions to obtain ethyl thioglycolate with a yield of 90%. The characterization data of the obtained ethyl thioglycolate product are the same as in Example 1.
Embodiment 3
[0020] Embodiment 3: in the round bottom flask of 100ml, add the mercaptoacetic acid of 27.6g (300mmol), the ethanol of 27.6g (600mmol), the cyclohexane of 16ml, the magnesium perchlorate of 6.69g (30mmol), under stirring Heat to reflux, water will continue to come out from the water separator, stop the reaction when no more water is separated, distill off excess alcohol and cyclohexane under normal pressure, then add 10ml of distilled water, and then extract with diethyl ether (10ml×3) , organic phase with MgSO 4 Dry, filter, remove ether, then distill under reduced pressure at 80-82°C / 1.3KPa-1.35KPa and collect fractions to obtain ethyl thioglycolate with a yield of 98%. The characterization data of the obtained ethyl thioglycolate product are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com