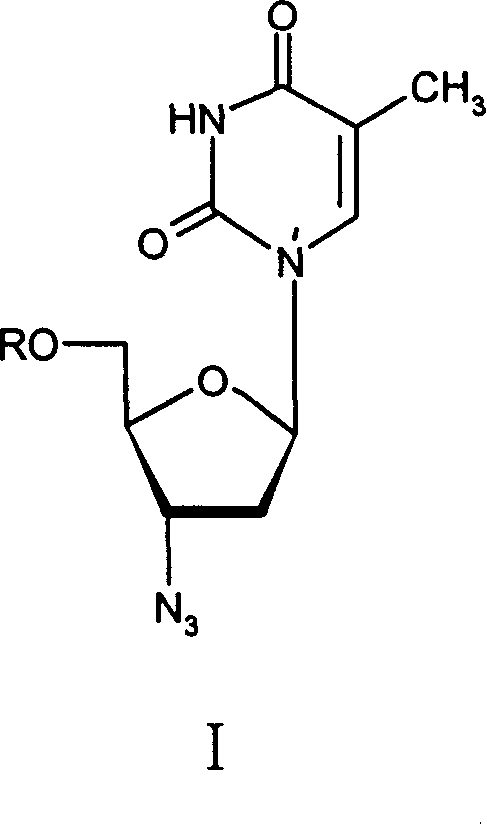
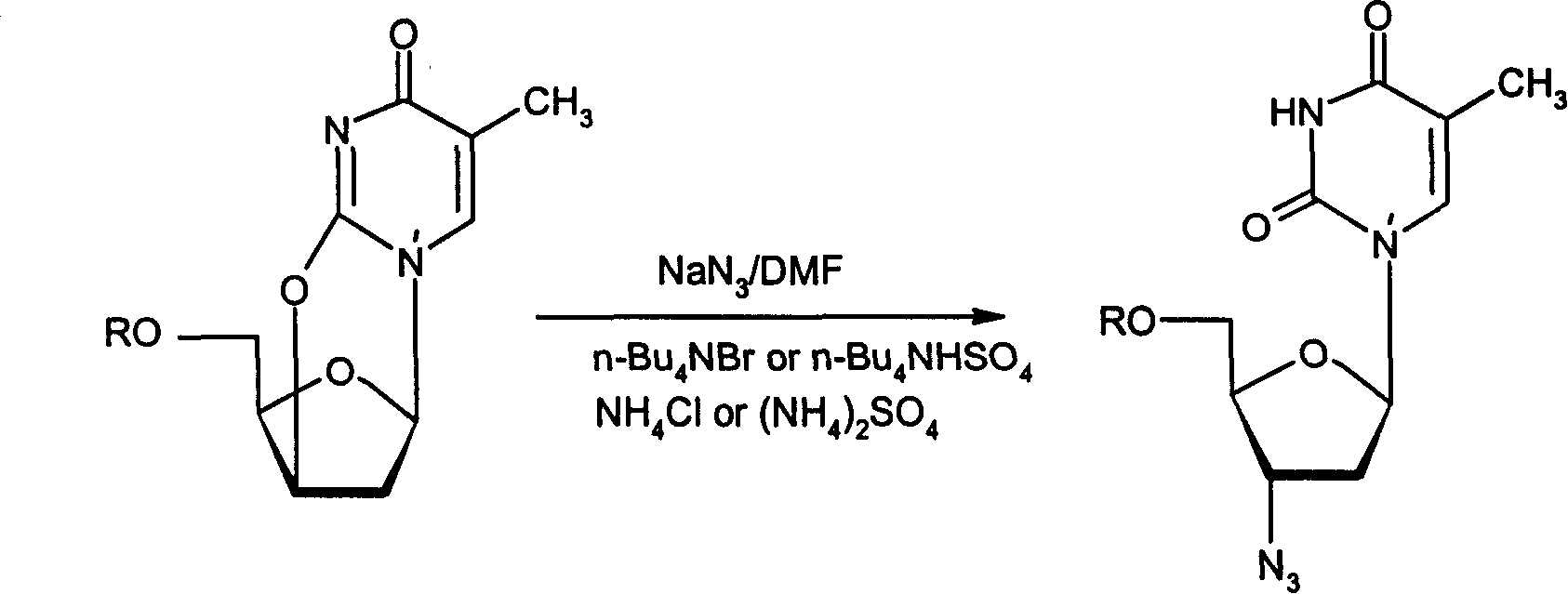Method for preparing Zidovudine azide intermediate by phase transition method
A zidovudine and phase transfer technology, applied in sugar derivatives, organic chemistry, etc., can solve the problems of easy explosion of azide reagents and long reaction time, and achieve the advantages of reducing hidden dangers in production, short reaction time and mild operating conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Under the protection of argon, dissolve 5g of zidovudine oxygen bridge in 100ml of DMF, then add 4.2g of sodium azide, 7g of tetrabutylammonium bromide, and 0.6g of ammonium chloride in sequence, stir evenly, and heat up Reflux at 80°C for 6 hours. After the reaction was completed, it was cooled to room temperature, filtered, and the filtrate was concentrated to dryness under reduced pressure. Add 100ml of dichloromethane and 50ml of water to the residue to dilute, separate the organic layer, back-extract the aqueous layer with 20ml×2 of dichloromethane, combine the organic layers, wash with 50ml×3 of water, and dry over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure, and dried in vacuum with phosphorus pentoxide to obtain 0.95 g of zidovudine azide intermediate, with a yield of 87%.
Embodiment 2
[0023] Under the protection of argon, dissolve 5g of Zidovudine bridge oxide in 100ml of DMF, then add 4.2g of sodium azide, 7g of tetrabutylammonium bromide, and 1.4g of ammonium sulfate in sequence, stir well, and heat up to 80 °C reflux for 6.5 hours. The reaction treatment was the same as in Example 1 to obtain 1.0 g of the product, with a yield of 91%.
Embodiment 3
[0025] Under the protection of argon, dissolve 5g of Zidovudine bridge oxide in 100ml of DMF, then add 4.2g of sodium azide, 7g of tetrabutylammonium bromide, 5.0g of 001*7 strong acid resin, and stir well , heated to 80°C and refluxed for 6 hours. The reaction treatment was the same as that in Example 1 to obtain 0.90 g of the product, with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com