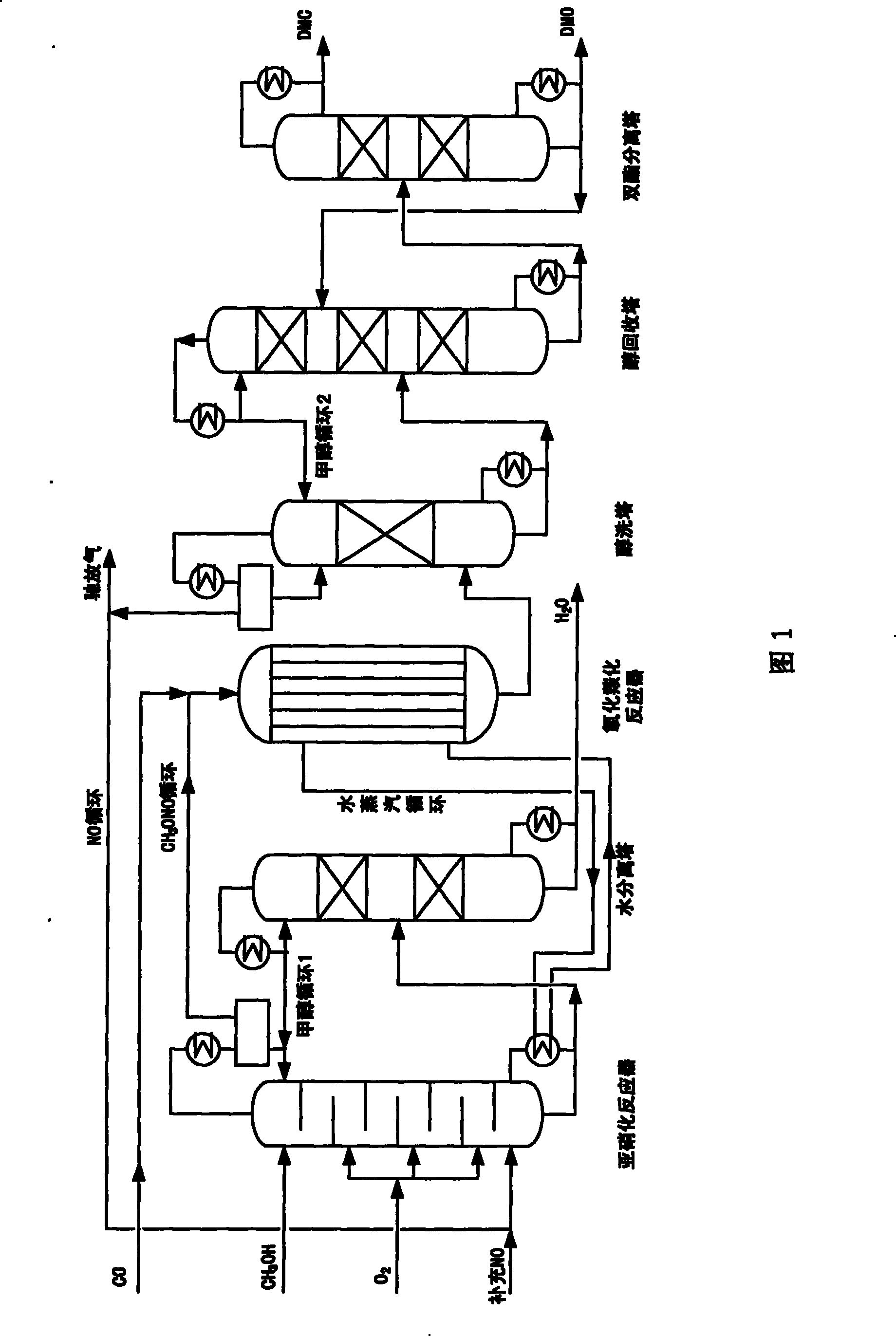
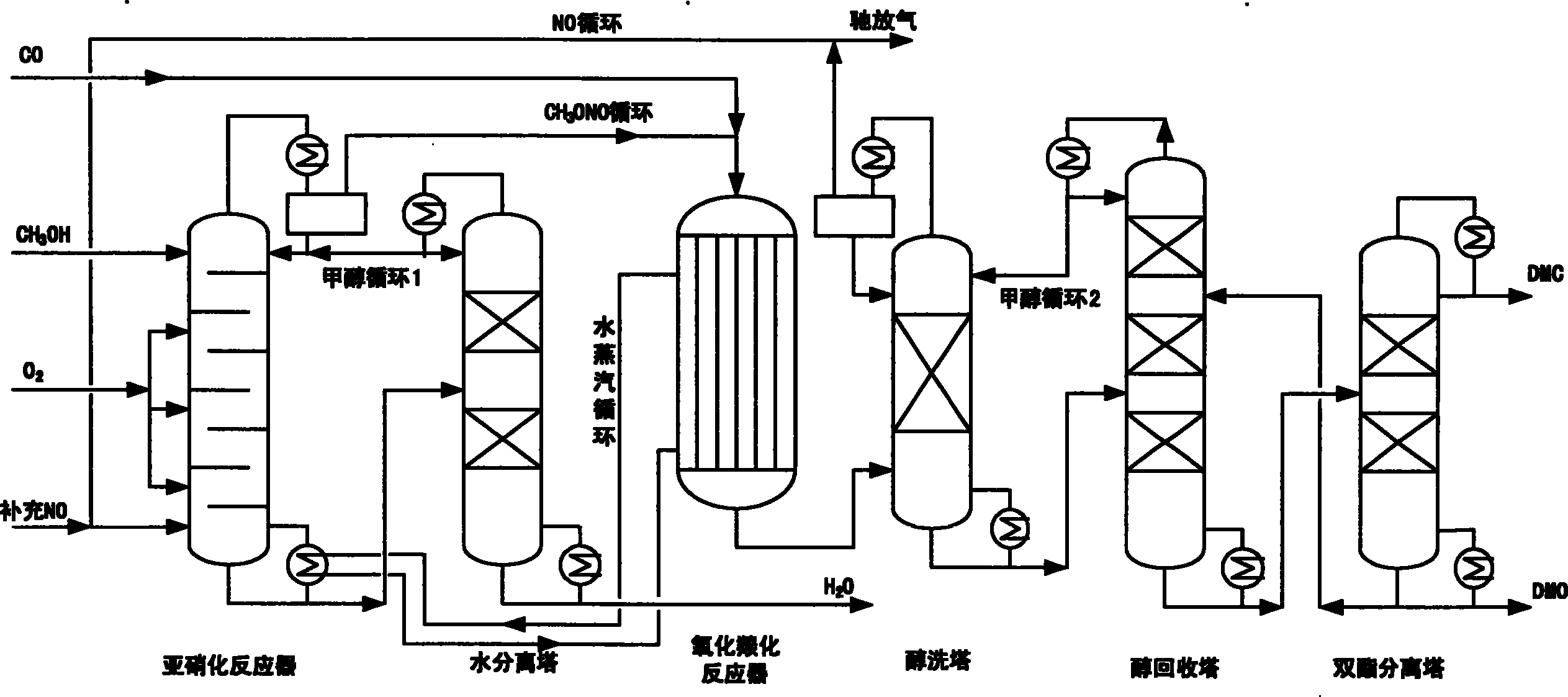
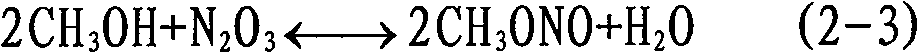Method for synthesizing dimethyl oxalate and coproducing dimethyl carbonate
A technology for synthesizing dimethyl oxalate and dimethyl carbonate, which is applied in the preparation of carbonate/haloformate, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as failure to handle well
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The present embodiment is the scale of annual output of 5000 tons of dimethyl oxalate.
[0084] CO is 98.5% pure, the rest as N 2 .
[0085] o 2 The purity is also 98.5%, the rest is N 2 .
[0086] The purity of methanol is 99.9%, and the rest is water.
[0087] Supplementing NO is supplemented by the method of generating methyl nitrite from sodium nitrite, sulfuric acid and methanol.
[0088] In this example, the composition of the purge gas is: NO = 15%, CO = 10%, nitrite = 2%, methanol = 8%, N 2 = 65%. From raw gas CO and O 2 N brought into the system 2 The exhausted NO loss is 0.052 kmol / h, the CO loss is 0.03 kmol / h, and the methanol is 0.03 kmol / h.
[0089] In addition, in the nitrosation reactor, the selectivity of producing nitric acid is 2%, therefore, the amount of nitric acid produced per hour is 0.17 kmol / h, and the equivalent loss of NO is 0.17 kmol / h. It can be seen that the total NO loss is 0.22kmol / h, which is supplemented by the reaction of so...
Embodiment 2
[0103] The present embodiment is the scale of annual production of 10,000 tons of dimethyl oxalate.
[0104] CO is 98.5% pure and the remainder is N2.
[0105] The O2 purity is also 98.5%, and the rest is N2.
[0106] The purity of methanol is 99.9%, and the rest is water.
[0107] The method of supplementing NO is supplemented by ammonia oxidation.
[0108] In this example, the composition of the purge gas was: NO = 10%, CO = 10%, nitrite = 1%, methanol = 5%, and N2 was 74%. The N2 brought into the system by the raw material gas CO and O2 is 0.4kmol / h, and it is discharged through the purge gas. The NO loss is 0.073kmol / h, the CO loss is 0.073kmol / h, and the methanol is 0.036kmol / h.
[0109] In addition, in the nitrosation reactor, the selectivity of producing nitric acid is 1.2%, therefore, the amount of nitric acid produced per hour is 0.2 kmol / h, and the equivalent loss of NO is 0.2 kmol / h. It can be seen that the total NO loss is 0.273kmol / h, supplemented by the ammon...
Embodiment 3
[0123] The present embodiment is the scale of annual production of 1000 tons of dimethyl oxalate.
[0124] CO is 98.5% pure and the remainder is N2.
[0125] The O2 purity is also 98.5%, and the rest is N2.
[0126] The purity of methanol is 99.9%, and the rest is water.
[0127] The method of supplementing NO is supplementing with purchased NO cylinder gas.
[0128] In this example, the composition of the purge gas is: NO = 20%, CO = 10%, nitrite = 3%, methanol = 10%, N2 is 57%. The N2 brought into the system by the raw material gas CO and O2 is 0.04kmol / h, and it is discharged through the purge gas. The NO loss is 0.016kmol / h, the CO loss is 0.007kmol / h, and the methanol is 0.007kmol / h.
[0129] In addition, in the nitrosation reactor, the selectivity of producing nitric acid is 2.0%, therefore, the amount of nitric acid produced per hour is 0.02 kmol / h, and the equivalent loss of NO is 0.02 kmol / h. It can be seen that the total NO loss is 0.036kmol / h, equivalent to 0.81...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com