Medicinal composition containing mongolian snakegourd and notoginseng
A composition, the technology of the extract of Trichosanthes mellifera, which is applied in the field of medicine to achieve the effects of good preparation stability, convenient product quality, and product quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
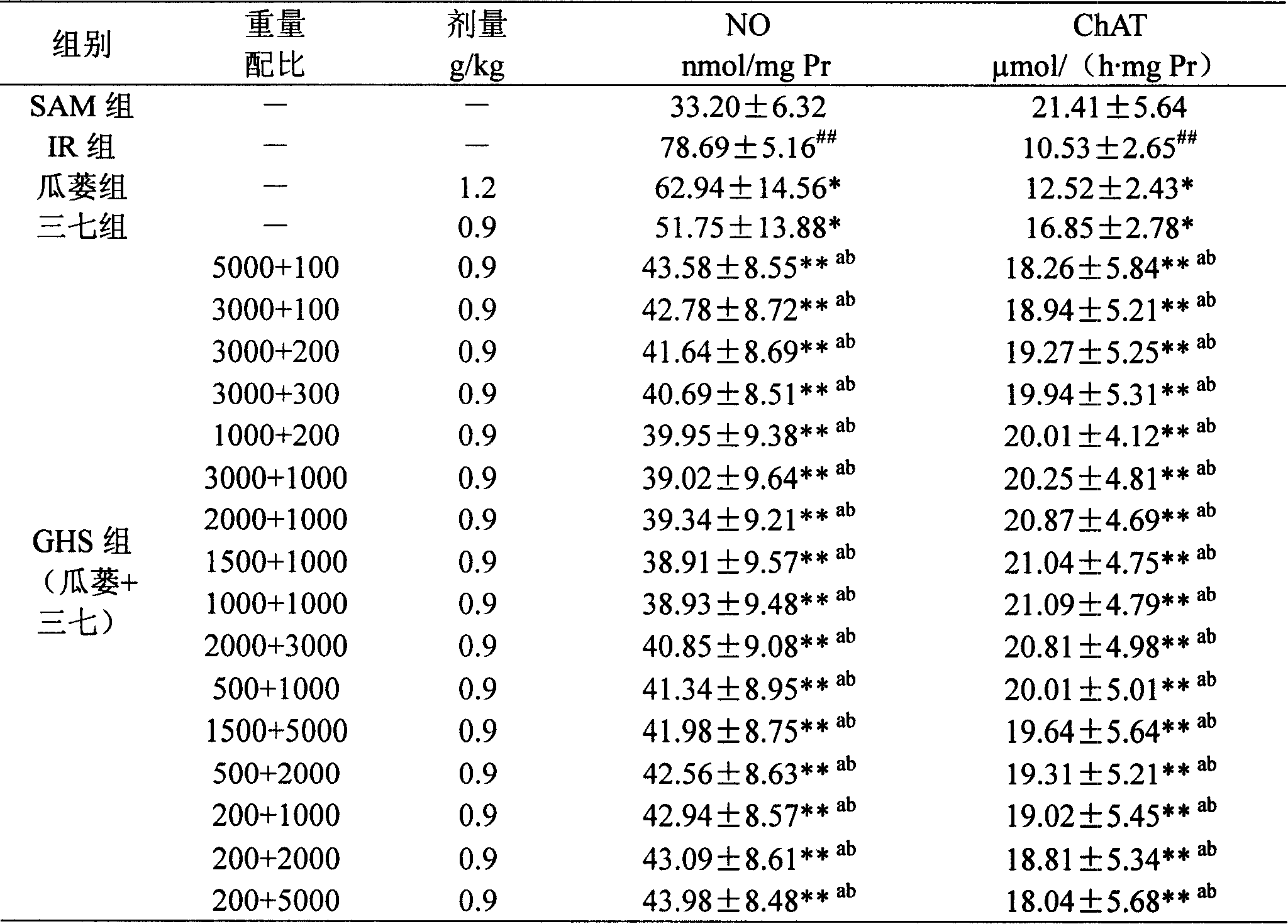
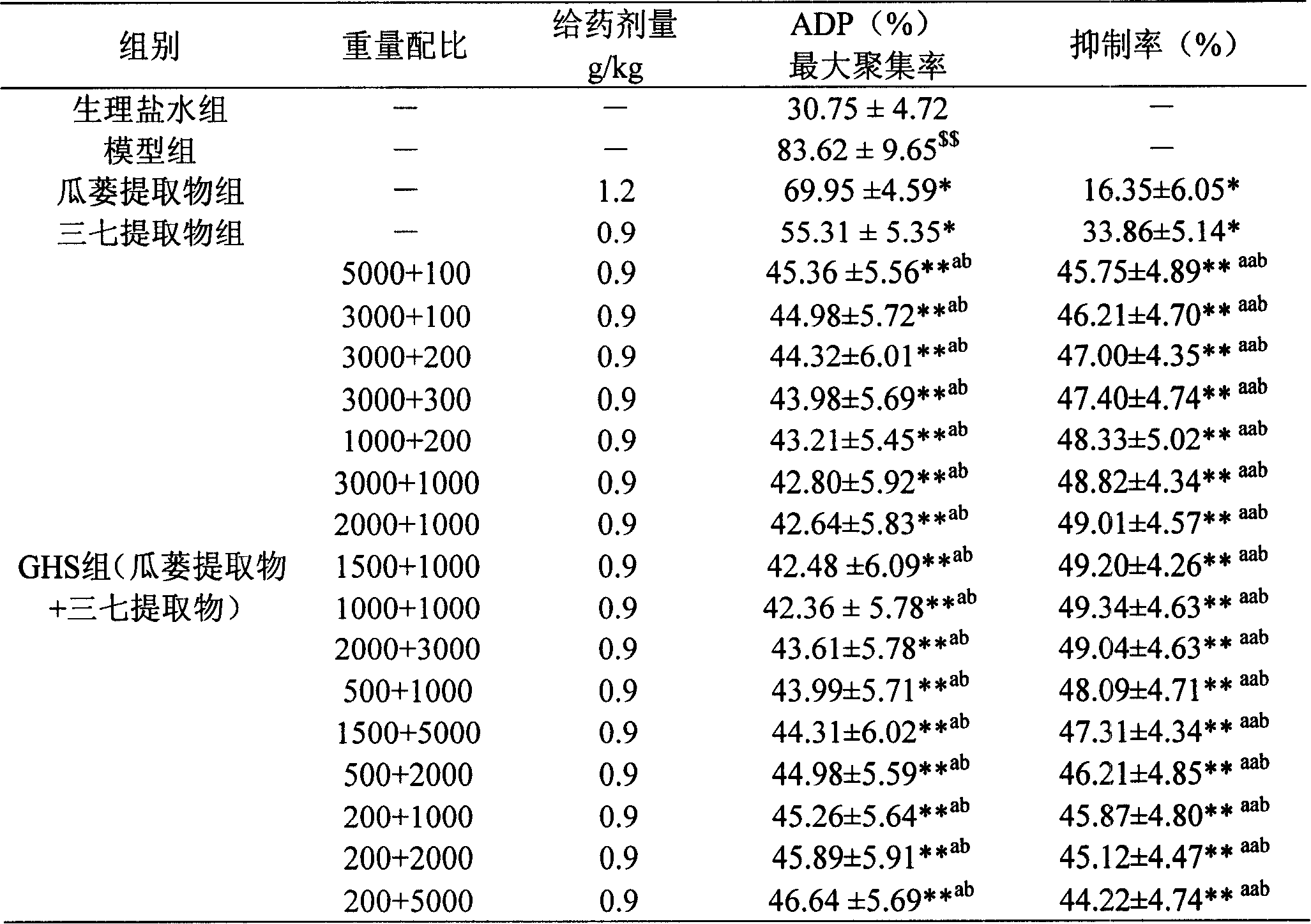
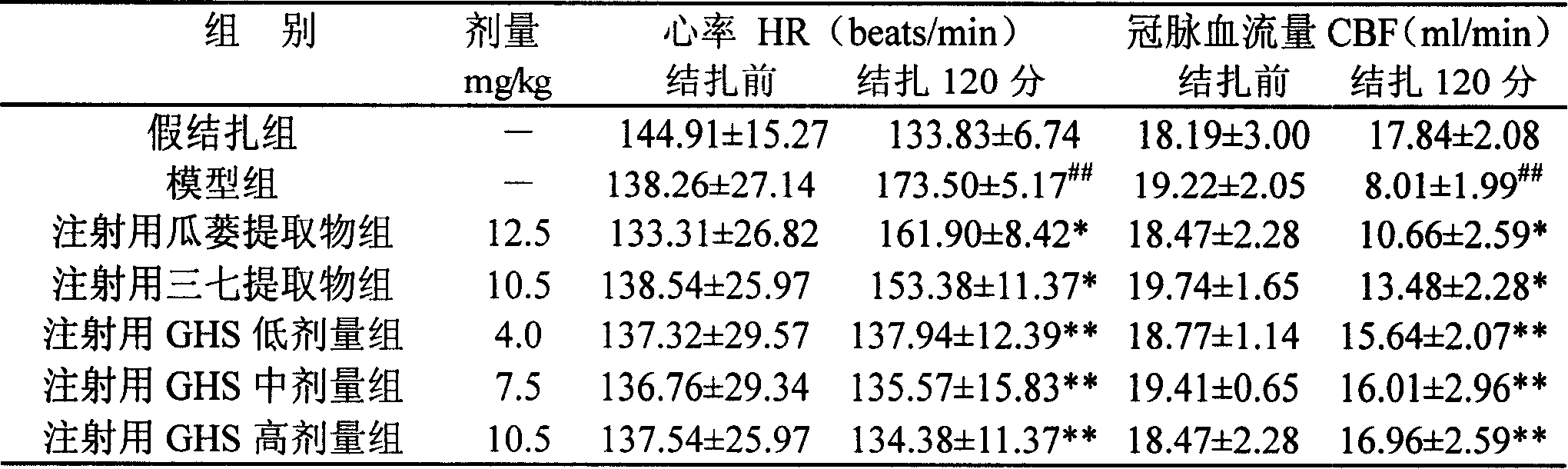
Image
Examples
Embodiment 1
[0087] Example 1 Preparation of Trichosanthes Fructus Extract
[0088] 1, the preparation method of Trichosanthes quince extract
[0089] Preparation of Trichosanthes extract:
[0090]Take Trichosanthes medicinal material, dry, crush into coarse powder, add water to reflux and extract twice, add 10 times the amount of water each time, reflux extract for 2 hours, filter, combine filtrates, concentrate to a relative density of 1.10-1.15 (60°C ) concentrated solution, add ethanol until the content reaches 80%, leave it overnight, take the supernatant overnight, recycle the ethanol until it has no alcohol smell, concentrate under reduced pressure to a thick paste, and vacuum dry to obtain the crude extract of Trichosanthes trichosanthes.
[0091] After dissolving the above-mentioned Trichosanthes quinquefolius extract with an appropriate amount of water, shake and extract twice with an equal amount of petroleum ether, shake and extract the water liquid three times with an equal...
Embodiment 2
[0099] Example 2 Preparation of Radix Notoginseng Extract
[0100] 1. The preparation method of Panax notoginseng extract
[0101] Take the notoginseng medicinal material, crush it into a coarse powder, add ethanol to reflux and extract three times, each time for 3 hours, filter, recover the ethanol from the filtrate until it has no alcohol smell, add water to an appropriate amount (each 1ml is equivalent to 2g of the original medicinal material), refrigerate for 24 hours, filter After that, the filtrate was added to the treated weakly polar macroporous resin column, first eluted with 2 times the column volume of water, then 4 to 5 times the 80% ethanol, collected the eluate, recovered the ethanol and Vacuum drying, that is.
[0102] 2. Determination of the content of Panax notoginseng extract
[0103] Determination according to high performance liquid chromatography (Appendix VI D)
[0104] Chromatographic conditions and system adaptability test: Octadecylsilane bonded s...
Embodiment 3
[0112] The preparation of embodiment 3 GHS tablet
[0113] Prescription one:
[0114] Trichosanthes extract 71g (equivalent to 1000g of raw medicinal materials)
[0115] Panax notoginseng extract 8.4g (equivalent to 200g of raw medicinal materials)
[0116] 120g pregelatinized starch
[0117] Microcrystalline Cellulose 40g
[0118] Low-substituted hydroxypropyl cellulose 40g
[0119] 2% HPMC aqueous solution appropriate amount
[0121] Carboxymethyl Starch Sodium 12g
[0122]
[0123] A total of 1000 tablets were prepared
[0124] Prescription two:
[0125] Trichosanthes extract 71g (equivalent to 1000g of raw medicinal materials)
[0126] Panax notoginseng extract 12.6g (equivalent to 300g of raw medicinal materials)
[0127] 120g pregelatinized starch
[0128] Microcrystalline Cellulose 40g
[0129] Low-substituted hydroxypropyl cellulose 40g
[0130] 2% HPMC aqueous solution approp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com