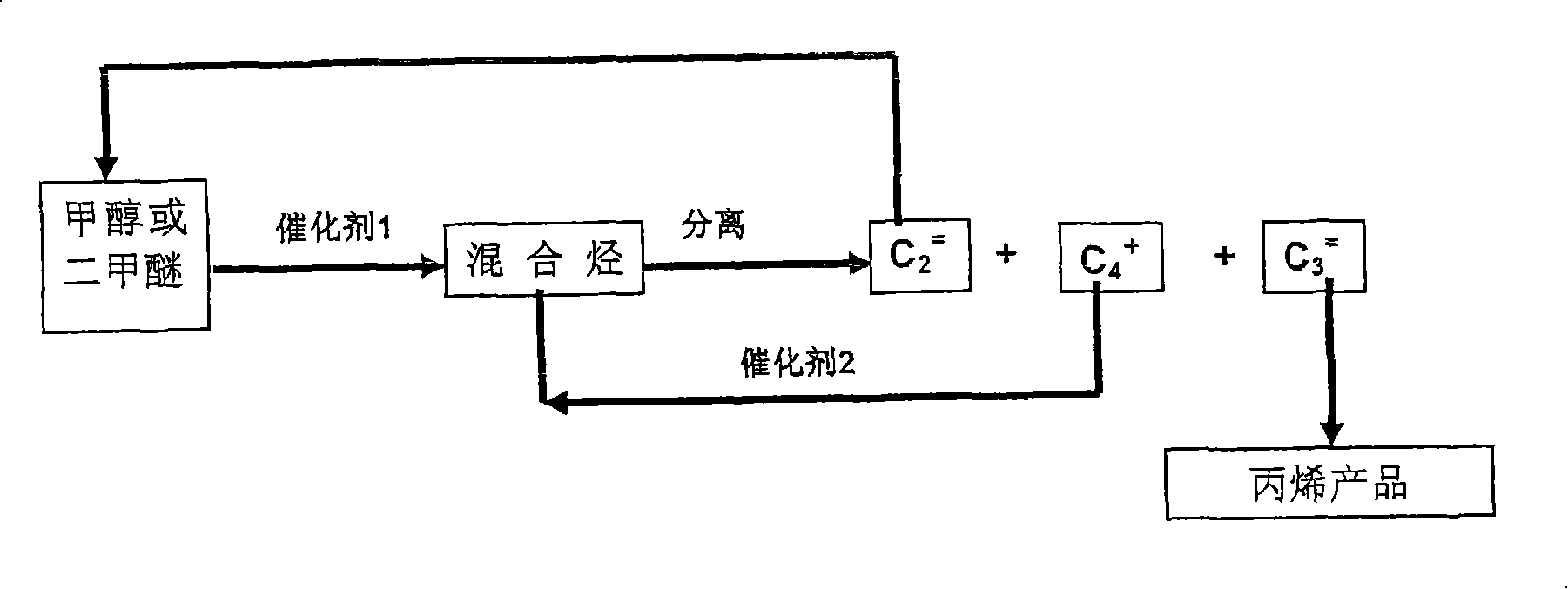
Method for producing propylene by carbinol or dimethyl ether
A technology of dimethyl ether and methanol, applied in the field of highly selective preparation of propylene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] SiO 2 / Al 2 o 3 =200 ZSM-5 zeolite is the active component, and the first catalyst for methanol conversion is prepared by leaching La, Mg, spray drying, water treatment, online silanization and other processes. A fluidized bed reaction process is adopted, and 70% methanol aqueous solution is used as a raw material. The fluidized bed reaction conditions are: temperature 502°C, pressure (gauge pressure) 0.03MPa, methanol feed / first catalyst circulation rate 1.0, space velocity (calculated as pure methanol) 5h -1, the residence time of the first catalyst in the reactor is 90min, the residence time of the reaction gas is 5s, and the regeneration temperature of the first catalyst is 620°C. The reaction result is as follows: methanol conversion rate 99.2%, product distribution: methane 0.61%, carbon oxide (CO+CO 2 ) 0.83%, ethane + propane 1.03%, ethylene 5.24%, propylene 50.10%, C 4 The above alkanes and alkenes are 38.94%, and aromatics are 3.25%.
[0032] According t...
Embodiment 2
[0036] Using ZSM-5 as the active component, with Al 2 o 3 As a binder, the oil injection molding process is used to make a moving bed catalyst, and the catalyst is modified to make the first moving bed catalyst for methanol conversion to propylene. Using moving bed reaction process, 70% methanol aqueous solution as raw material, reaction temperature is 480°C, pressure (gauge pressure) 0.1MPa, methanol feeding space velocity (calculated as pure methanol) 5h -1 , the average residence time of the first catalyst in the reactor is 30h, and the residence time of the reaction gas is 3s. Reaction result: the conversion rate of methanol is 99.6%. Product distribution: methane 0.37%, carbon oxides (CO+CO 2 ) 0.63%, ethane + propane 0.96%, ethylene 4.39%, propylene 55.07%, C 4 The above alkanes and alkenes are 36.40%, and aromatics are 2.18%.
[0037] Pass methanol and light components (mixed light components according to the material composition after methanol conversion, includin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com