Method for measuring enzymatic activity by integration method and initial rate method
A technology of initial velocity and integral method, which is applied in the field of determination of enzyme activity by combining integral method and initial velocity method, can solve problems such as error, random error system, and not reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
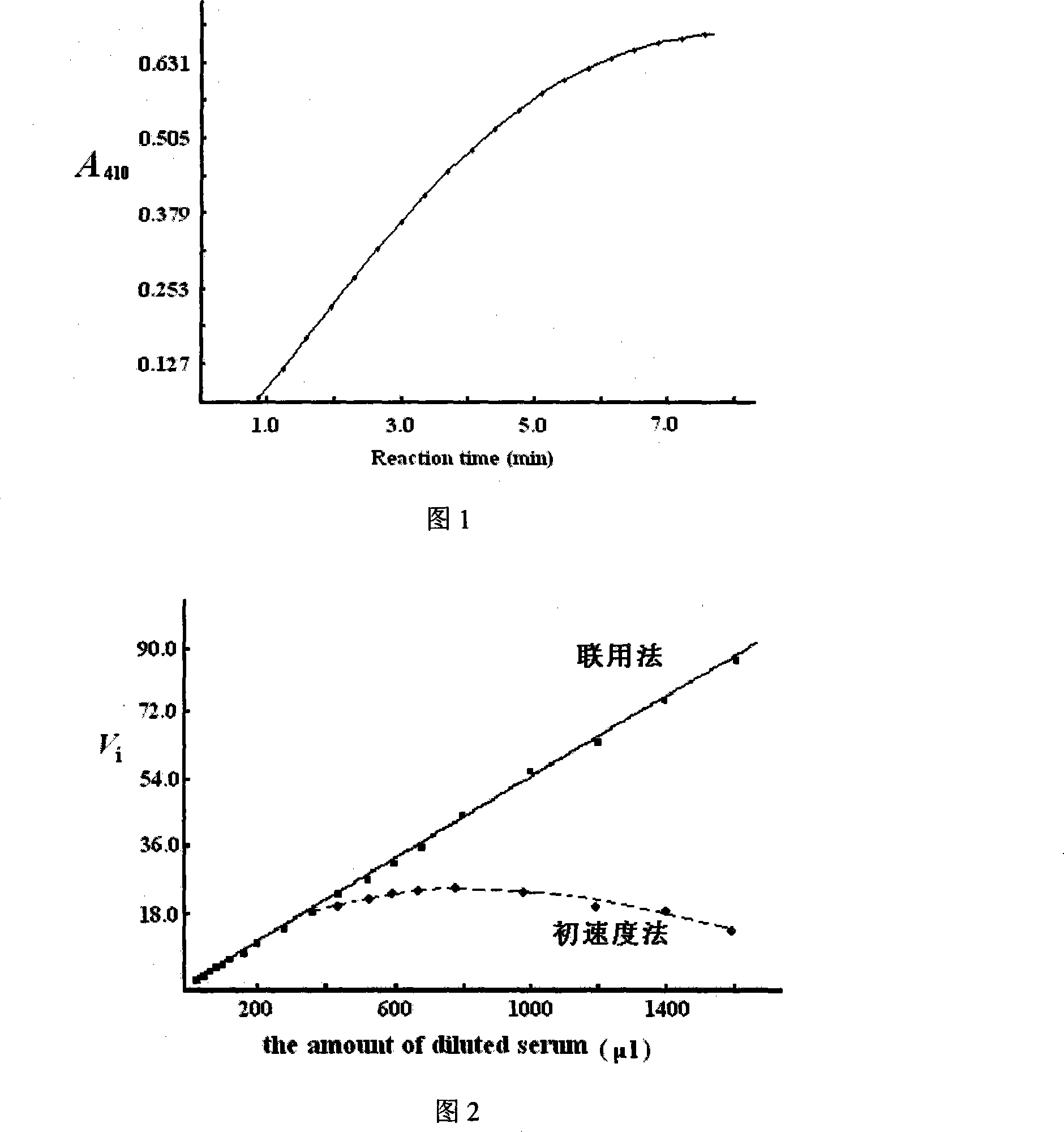
[0034] Embodiment 1: this combined method can measure human serum butyrylcholinesterase activity, it is characterized in that carry out as follows:
[0035] (1), add 50mmol / L sodium phosphate buffer solution (pH 7.4) totally 0.90ml in test tube, and the thiobis(2-nitrobenzoic acid, Dithiobis-(2-nitrobenzoic acid) dissolved in this phosphate buffer solution acid), DTNB) in total 0.10ml (final concentration 0.50mmol / L), 0.10ml of an appropriately diluted serum sample, keep the temperature in a water bath at 30°C for 5 minutes;
[0036] (2) Add 100 μl of the substrate thiobutyrylcholine dissolved in the above-mentioned phosphate buffer solution to a final concentration of 54 μmol / L, and vortex to mix thoroughly;
[0037] (3), transfer the reaction mixture into the cuvette quickly, delay for 15 seconds, continuously monitor the 410nm absorption change at intervals of 5 seconds, and monitor for 5.0 minutes in total to obtain the reaction curve of the reaction product absorption inc...
Embodiment 2
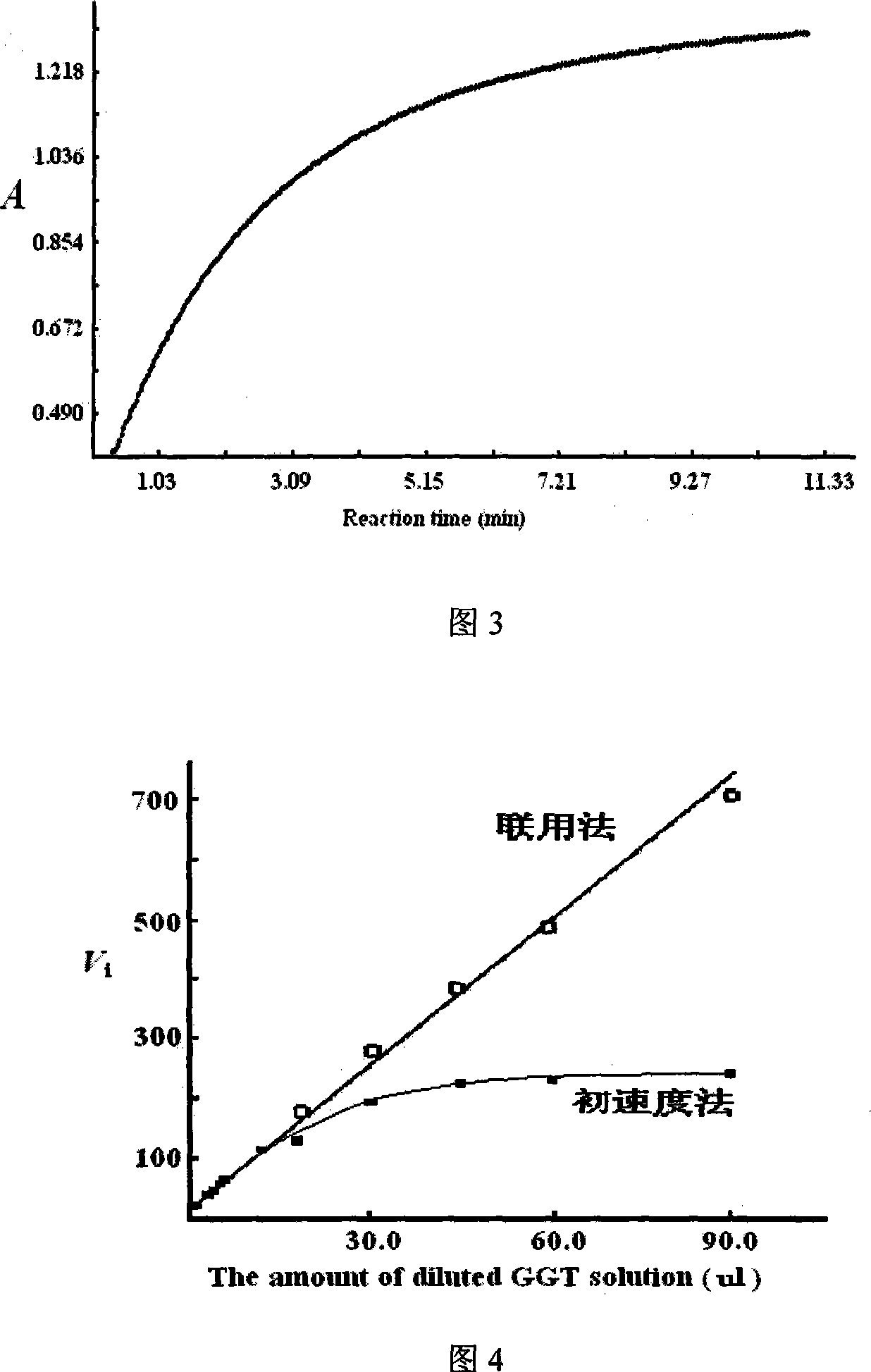
[0045] Embodiment 2: This combined method can measure the gamma-glutamyl transferase activity in mouse kidney or human serum, it is characterized in that carry out as follows:
[0046] (1) Add 110 μmol / L of L-γ-glutamyl p-nitroaniline, 15.0 mmol / L of diglycine, and MgCl to the test tube. 2 10.0mmol / L Tris-HCl buffer solution (100mmol / L, pH 8.1) 1.10ml in total; keep the temperature in a water bath at 30°C for 5 minutes;
[0047] (2) Take 100 μl of an appropriate diluted sample and add it to the above-mentioned test tube, mix well so that the final concentration of the initial substrate is 100 μmol / L;
[0048] (3), transfer the reaction mixture into the cuvette rapidly, delay for 15 seconds, continuously monitor the 405nm absorption change at an interval of 5 seconds, and monitor for 10.0 minutes in total to obtain the reaction curve (accompanying drawing 3) of product p-nitroaniline absorption increase;
[0049] (4) Delete the data whose light absorption increases by less tha...
Embodiment 3
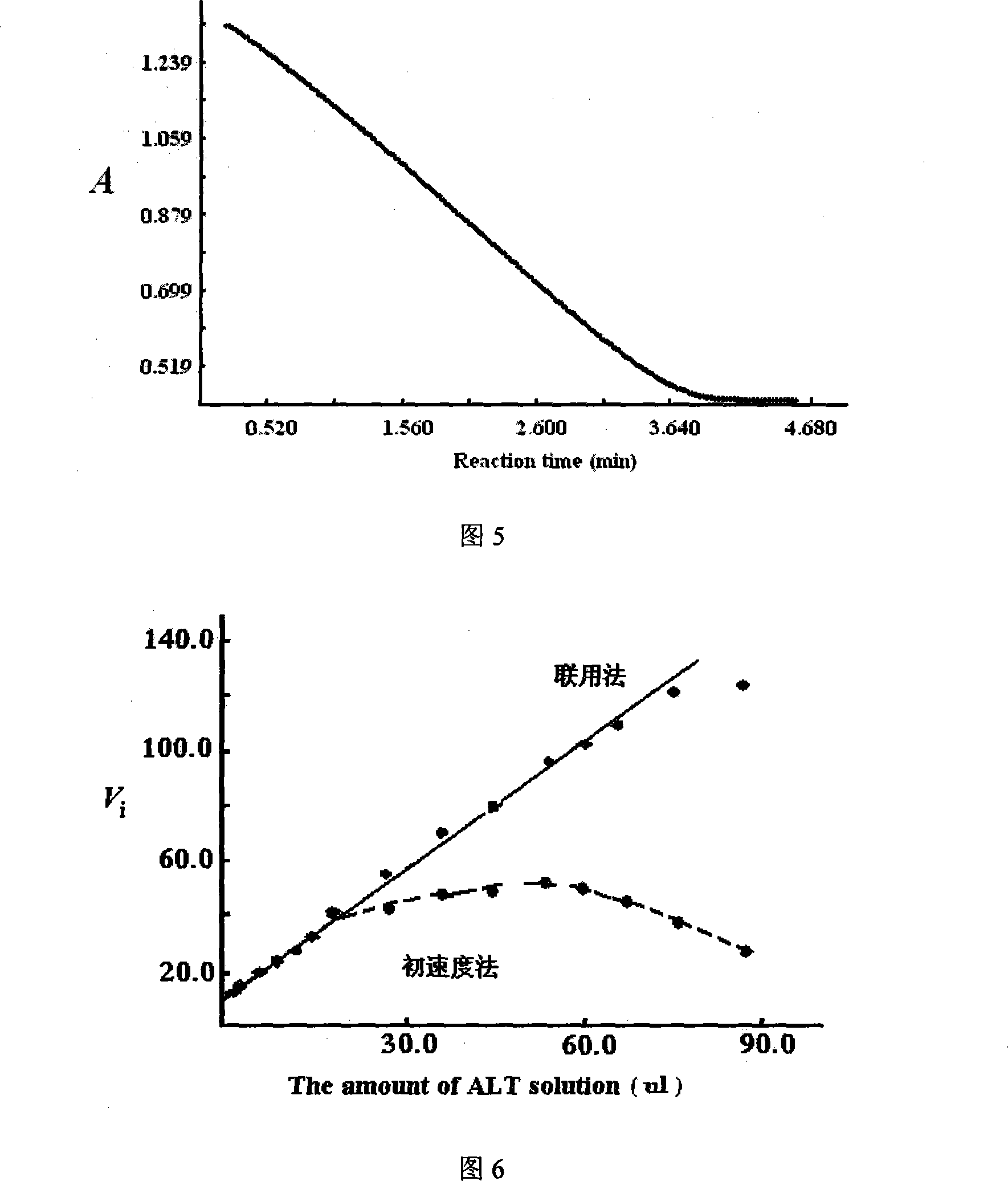
[0056] Embodiment 3: the characteristic steps of this combination method measuring mouse kidney, human serum alanine aminotransferase (ALT) activity are as follows:
[0057] (1), add 54 μmol / L of L-γ-glutamyl p-nitroaniline containing 600mmol / L alanine, 0.22mmol / L NADH, 1400U / L LDH, 100mmol / L Tris-HCl, bis Glycine 15.0mmol / L, MgCl2 10.0mmol / L, buffer solution (pH 7.3) containing 100mmol / L Tris-HCl 1.00ml in total; then add 100μl of properly diluted sample, mix quickly and in 30 ℃ constant temperature for 5 minutes;
[0058] (2) Add 100 μl of 16.3 mmol / L α-ketoglutarate solution into the above-mentioned test tube, shake and mix quickly;
[0059] (3), transfer the reaction mixture into the cuvette rapidly, delay for 30 seconds, continuously monitor the 340nm absorption change at 1 second intervals, and monitor for 5.0 minutes in total to obtain a reaction curve indicating that the instantaneous absorption (Ai) of the substrate (NADH) drops ( Accompanying drawing 5);
[0060] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com